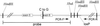Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis - PubMed (original) (raw)
Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis
Robert E Fleming et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Hereditary hemochromatosis (HH) is a common genetic disorder characterized by excess absorption of dietary iron and progressive iron deposition in several tissues, particularly liver. The vast majority of individuals with HH are homozygous for mutations in the HFE gene. Recently a second transferrin receptor (TFR2) was discovered, and a previously uncharacterized type of hemochromatosis (HH type 3) was identified in humans carrying mutations in the TFR2 gene. To characterize the role for TFR2 in iron homeostasis, we generated mice in which a premature stop codon (Y245X) was introduced by targeted mutagenesis in the murine Tfr2 coding sequence. This mutation is orthologous to the Y250X mutation identified in some patients with HH type 3. The homozygous Tfr2(Y245X) mutant mice showed profound abnormalities in parameters of iron homeostasis. Even on a standard diet, hepatic iron concentration was several-fold higher in the homozygous Tfr2(Y245X) mutant mice than in wild-type littermates by 4 weeks of age. The iron deposition in the mutant mice was predominantly hepatocellular and periportal. The mean splenic iron concentration in the homozygous Tfr2(Y245X) mutant mice was significantly less than that observed in the wild-type mice. The homozygous Tfr2(Y245X) mutant mice also demonstrated elevated transferrin saturations. There were no significant differences in parameters of erythrocyte production including hemoglobin levels, hematocrits, erythrocyte indices, and reticulocyte counts. Heterozygous Tfr2(Y245X) mice did not differ in any measured parameter from wild-type mice. This study confirms the important role for TFR2 in iron homeostasis and provides a tool for investigating the excess iron absorption and abnormal iron distribution in iron-overload disorders.
Figures
Fig 1.
Tfr2 Y245X allele. The genomic region surrounding exon 6 of the Tfr2 Y245X allele is represented. The position of the single C-to-G nucleotide substitution is demonstrated. The hatched region of intron 6 downstream of the _Xba_I site represents the retained polylinker sequence and loxP element from the transgene construct. The retained sequence includes two _Hin_dIII sites that allow distinguishing the wild-type and mutant alleles by Southern blot using the designated _Hin_dIII-to-_Xba_I genomic fragment as a probe. The mutant allele can be identified also by PCR using oligos at the designated positions, which gives a longer product from the mutant than from the wild-type allele.
Fig 2.
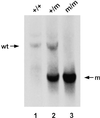
Southern blot analysis of genomic DNA from wild-type (wt) and Tfr2 Y245X mutant mice. A representative Southern blot of _Hin_dIII-digested genomic DNA isolated from tail samples from a wild-type (+/+), a Tfr2 Y245X heterozygous (+/m), and a homozygous (m/m) mouse and probed with the 32P-labeled _Hin_dIII-to-_Xba_I genomic DNA fragment designated in Fig. 1. A 4.3-kb restriction fragment is evident from the wild-type allele and a 2.3-kb fragment from the mutant allele.
Fig 3.
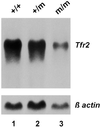
Northern blot analysis of Tfr2 mRNA expression in liver of wild-type and Tfr2 Y245X mutant mice. Fifteen micrograms of total cellular RNA from liver tissue of a wild-type (+/+), a heterozygous Tfr2 Y245X mutant (+/m), and a homozygous mutant (m/m) mouse were hybridized with a probe for mouse Tfr2. The blot was rehybridized with a probe for β-actin (Lower).
Fig 4.
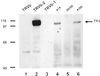
Western blot analysis of Tfr2 protein in hepatic membranes of wild-type and Tfr2 Y245X mutant mice. Whole-cell homogenates from the designated cell lines (lanes 1–3) or hepatic membrane preparations from the designated mice (lanes 4–6) were subjected to SDS/PAGE under reducing conditions, blotted, and reacted with an antibody to the intracellular domain of Tfr2.
Fig 5.
Hepatic iron concentrations in wild-type and Tfr2 Y245X mutant mice. Nonheme iron concentrations (μg/gram, dry weight) from 4-week-old wild-type (+/+), heterozygous Tfr2 Y245X (+/m), and homozygous Tfr2 Y245X (m/m) littermates are presented as mean ± SEM. *, P < 0.001 vs. each other group.
Fig 6.
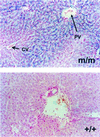
Perls' staining of liver sections. Representative sections from a homozygous Tfr2 Y245X (m/m) and wild-type (+/+) mouse stained for iron with the Perls' Prussian blue technique are shown. Staining is hepatocellular and predominantly periportal in the homozygous Tfr2 Y245X mouse. CV, central vein (short arrow); PV, portal vein (long arrow).
Fig 7.
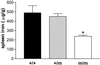
Splenic iron concentrations in wild-type and Tfr2 Y245X mutant mice. Nonheme iron concentrations (μg/gram, dry weight) from 4-week-old wild-type (+/+), heterozygous Tfr2 Y245X (+/m), and homozygous Tfr2 Y245X (m/m) littermates are presented as mean ± SEM. *, P < 0.001 vs. each other group.
Similar articles
- Iron absorption and hepatic iron uptake are increased in a transferrin receptor 2 (Y245X) mutant mouse model of hemochromatosis type 3.
Drake SF, Morgan EH, Herbison CE, Delima R, Graham RM, Chua AC, Leedman PJ, Fleming RE, Bacon BR, Olynyk JK, Trinder D. Drake SF, et al. Am J Physiol Gastrointest Liver Physiol. 2007 Jan;292(1):G323-8. doi: 10.1152/ajpgi.00278.2006. Epub 2006 Aug 24. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 16935854 - Transferrin receptor 2: continued expression in mouse liver in the face of iron overload and in hereditary hemochromatosis.
Fleming RE, Migas MC, Holden CC, Waheed A, Britton RS, Tomatsu S, Bacon BR, Sly WS. Fleming RE, et al. Proc Natl Acad Sci U S A. 2000 Feb 29;97(5):2214-9. doi: 10.1073/pnas.040548097. Proc Natl Acad Sci U S A. 2000. PMID: 10681454 Free PMC article. - Disruption of hemochromatosis protein and transferrin receptor 2 causes iron-induced liver injury in mice.
Delima RD, Chua AC, Tirnitz-Parker JE, Gan EK, Croft KD, Graham RM, Olynyk JK, Trinder D. Delima RD, et al. Hepatology. 2012 Aug;56(2):585-93. doi: 10.1002/hep.25689. Epub 2012 Jun 11. Hepatology. 2012. PMID: 22383097 - The mechanisms of systemic iron homeostasis and etiology, diagnosis, and treatment of hereditary hemochromatosis.
Kawabata H. Kawabata H. Int J Hematol. 2018 Jan;107(1):31-43. doi: 10.1007/s12185-017-2365-3. Epub 2017 Nov 13. Int J Hematol. 2018. PMID: 29134618 Review. - Mechanisms of iron accumulation in hereditary hemochromatosis.
Fleming RE, Sly WS. Fleming RE, et al. Annu Rev Physiol. 2002;64:663-80. doi: 10.1146/annurev.physiol.64.081501.155838. Annu Rev Physiol. 2002. PMID: 11826284 Review.
Cited by
- Of mice and men: the iron age.
Vaulont S, Lou DQ, Viatte L, Kahn A. Vaulont S, et al. J Clin Invest. 2005 Aug;115(8):2079-82. doi: 10.1172/JCI25642. J Clin Invest. 2005. PMID: 16075054 Free PMC article. - Non-HFE hemochromatosis: genetics, pathogenesis, and clinical management.
Nelson JE, Kowdley KV. Nelson JE, et al. Curr Gastroenterol Rep. 2005 Feb;7(1):71-80. doi: 10.1007/s11894-005-0069-y. Curr Gastroenterol Rep. 2005. PMID: 15701302 Review. - Hereditary hemochromatosis and transferrin receptor 2.
Chen J, Enns CA. Chen J, et al. Biochim Biophys Acta. 2012 Mar;1820(3):256-63. doi: 10.1016/j.bbagen.2011.07.015. Epub 2011 Aug 16. Biochim Biophys Acta. 2012. PMID: 21864651 Free PMC article. Review. - Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis.
Kohyama M, Ise W, Edelson BT, Wilker PR, Hildner K, Mejia C, Frazier WA, Murphy TL, Murphy KM. Kohyama M, et al. Nature. 2009 Jan 15;457(7227):318-21. doi: 10.1038/nature07472. Epub 2008 Nov 26. Nature. 2009. PMID: 19037245 Free PMC article. - Is the iron regulatory hormone hepcidin a risk factor for alcoholic liver disease?
Harrison-Findik DD. Harrison-Findik DD. World J Gastroenterol. 2009 Mar 14;15(10):1186-93. doi: 10.3748/wjg.15.1186. World J Gastroenterol. 2009. PMID: 19291818 Free PMC article. Review.
References
- Kawabata H., Yang, R., Hirama, T., Vuong, P. T., Kawano, S., Gombart, A. F. & Koeffler, H. P. (1999) J. Biol. Chem. 274, 20826-20832. - PubMed
- Kawabata H., Germain, R. S., Vuong, P. T., Nakamaki, T., Said, J. W. & Koeffler, H. P. (2000) J. Biol. Chem. 275, 16618-16625. - PubMed
- West A. P., Bennett, M. J., Sellers, V. M., Andrews, N. C., Enns, C. A. & Bjorkman, P. J. (2000) J. Biol. Chem. 275, 38135-38138. - PubMed
- Kawabata H., Germain, R. S., Ikezoe, T., Tong, X., Green, E. M., Gombart, A. F. & Koeffler, H. P. (2001) Blood 98, 1949-1954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HL66225/HL/NHLBI NIH HHS/United States
- R01 DK053405/DK/NIDDK NIH HHS/United States
- R01 DK040163/DK/NIDDK NIH HHS/United States
- R01DK40163/DK/NIDDK NIH HHS/United States
- R01 GM034182/GM/NIGMS NIH HHS/United States
- R01DK34182/DK/NIDDK NIH HHS/United States
- R01 HL066225/HL/NHLBI NIH HHS/United States
- R01DK53405/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials