Rac regulates endothelial morphogenesis and capillary assembly - PubMed (original) (raw)
Rac regulates endothelial morphogenesis and capillary assembly
John O Connolly et al. Mol Biol Cell. 2002 Jul.
Abstract
Endothelial cells undergo branching morphogenesis to form capillary tubes. We have utilized an in vitro Matrigel overlay assay to analyze the role of the cytoskeleton and Rho GTPases during this process. The addition of matrix first induces changes in cell morphology characterized by the formation of dynamic cellular protrusions and the assembly of discrete aggregates or cords of aligned cells resembling primitive capillary-like structures, but without a recognizable lumen. This is followed by cell migration leading to the formation of a complex interconnecting network of capillary tubes with readily identifiable lumens. Inhibition of actin polymerization or actin-myosin contraction inhibits cell migration but has no effect on the initial changes in endothelial cell morphology. However, inhibition of microtubule dynamics prevents both the initial cell shape changes as well as cell migration. We find that the small GTPase Rac is essential for the matrix-induced changes in endothelial cell morphology, whereas p21-activated kinase, an effector of Rac, is required for cell motility. We conclude that Rac integrates signaling through both the actin and microtubule cytoskeletons to promote capillary tube assembly.
Figures
Figure 1
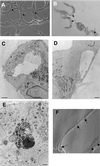
Phase contrast and electron micrographs of capillary tube formation. (A) Endothelial cells overlaid with Matrigel form a network of capillary tube-like structures composed of multiple cells with intercellular spaces or lumens. Cells are bipolar and are aligned in the axis of the tube. (B) High resolution phase contrast of a 1-μm transverse section through capillary-like tubes demonstrating lumen formation (arrows). (C) Electron micrograph demonstrating lumen in capillary tube. Lumens frequently contain debris or matrix-like material. (D) Some capillary tubes are composed of single endothelial cells containing a large vacuole or lumen. (E) Apoptotic cell surrounded by three cells and detached from matrix. (F) Modulation contrast image of capillary tube with vacuoles (arrows) and intercellular spaces (arrowheads). Bar, 100 μm in A, and 1 μm in C-E.
Figure 2
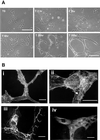
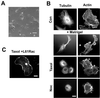
Morphological changes during capillary tube formation. (A) Phase contrast micrographs showing cell morphology changes during capillary tube formation. Final panel is a higher resolution view of mature tubules. Bar, 100 μm. (B) Endothelial cells were fixed and labeled with rhodamine phalloidin (i and ii), anti-VE cadherin antibody (iii), and anti-tyr-tubulin antibody (iv). Capillary tube structures had little organized filamentous actin structures and formed branching networks, with some cells extending protrusions along one another (arrow in iii). Cells formed adherens junctions (iii), and microtubules aligned along the long axis of the tubule (iv). Bar, 50 μm.
Figure 3
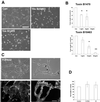
Rho is not required for capillary tube formation. (A) Cells were treated with the indicated concentration of toxin B 10463 or 1470 and were overlaid with Matrigel. (B) Capillary tube formation was inhibited in a dose-dependent manner. Results represent mean ± SD from at least three independent experiments performed in duplicate. *P < 0.01, **P < 0.001 in unpaired t test. Bar, 100 μm. (C) Cells were treated with the Rho kinase inhibitor Y-27632 (10 μM). Capillary tube formation was not affected, and intercellular spaces (arrows) resembling lumens were readily seen (upper panels). Cells were labeled with rhodamine phalloidin after overnight treatment with Y-27361and after tube formation in the presence of the drug. Lamellipodia formation was not affected, but actin stress fiber formation was inhibited (lower panels). Bar, 50 μm. (D) Cells were treated with C3 exoenzyme (3 μg/ml) or Y27632 (10 μM) and were overlaid with Matrigel. Tube formation was assessed after 24 h. Data represents mean ± SD from experiments performed in duplicate at least four times. No significant differences by unpaired t test.
Figure 4
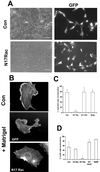
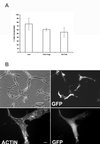
Rac is required for capillary morphogenesis. (A and C) Cells were injected with the indicated constructs and GFP vector. Injected cells incorporated into capillary-like structures composed of at least three cells were scored as positive. Cells injected with N17 Rac were not incorporated into capillary tubes. Results (mean ± SD) from at least four independent experiments performed in duplicate are shown. **P < 0.001 in unpaired t test. Bar, 100 μM. (B and D) Cells were microinjected with N17 Rac and GFP or GFP expression vector alone, overlaid with Matrigel, and fixed and stained with rhodamine phalloidin after 3 h. A cell without Matrigel overlay is shown for comparison (upper panel). Bar, 10 μm. (D) Cells were injected with the indicated constructs and the percentage that formed protrusions after Matrigel overlay is shown. Results show mean ± SD from least four experiments performed in duplicate. *P < 0.05 in unpaired t test.
Figure 5
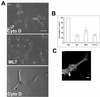
The actin cytoskeleton is not required for the early morphological response to Matrigel. (A and B) Cells were treated with Cytochalasin D (100 nM), ML7 (10 nM), and BDM (10 mM). Capillary tube formation was quantified 24 h after Matrigel overlay and results represent the mean ± SD from at least four independent experiments performed in duplicate. **P < 0.001 by unpaired t test. Bar 100, μm. Lower panel in A shows vacuole formation (arrows) still present in cells treated with cytochalasin D. (C) Cells treated with cytochalasin D and labeled with rhodamine phalloidin and antitubulin antibody. Protrusion formation was not affected by treatment with cytochalasin D. Bar, 10 μm.
Figure 6
The microtubule cytoskeleton is required for morphological response to Matrigel. (A**)** Cells treated with low-dose taxol (0.05 μM) do not form capillary-like structures. (B) Cells were labeled with antitubulin antibody and rhodamine phalloidin. Top panel shows growing HUVECs. Bottom panels show cells 3 h after Matrigel overlay either untreated or treated with taxol (0.05 μM) or Nocodazole (0.1 μM). Bar, 10 μM. (C) Taxol-treated cells injected with L61 Rac and overlaid with Matrigel. L61 Rac restored membrane ruffling but not protrusion formation. Bar, 10 μm in B and C.
Figure 7
Pak is required for endothelial motility but not the morphological response to Matrigel. (A) Cells were microinjected with the Pak inhibitory fragment (amino acids 83–149), kinase dead full-length Pak, or control vector and E-GFPC1. The number of injected cells incorporated into capillary structures is shown in and represents mean ± SD from at least three experiments performed in duplicate. No significant difference was detected (unpaired t test). (B) Cells were injected with Pak inhibitory fragment and photographed 24 h after Matrigel overlay (upper panels) or were fixed and stained with rhodamine phalloidin to demonstrate cell morphology (lower panels). Injected cells were incorporated into capillary-like structures and adopted an elongated bipolar morphology. Bar, 100 μm in upper panels and 10 μm in lower panels.
Similar articles
- Rho family GTPases regulate VEGF-stimulated endothelial cell motility.
Soga N, Namba N, McAllister S, Cornelius L, Teitelbaum SL, Dowdy SF, Kawamura J, Hruska KA. Soga N, et al. Exp Cell Res. 2001 Sep 10;269(1):73-87. doi: 10.1006/excr.2001.5295. Exp Cell Res. 2001. PMID: 11525641 - Distinct signals via Rho GTPases and Src drive shape changes by thrombin and sphingosine-1-phosphate in endothelial cells.
Vouret-Craviari V, Bourcier C, Boulter E, van Obberghen-Schilling E. Vouret-Craviari V, et al. J Cell Sci. 2002 Jun 15;115(Pt 12):2475-84. doi: 10.1242/jcs.115.12.2475. J Cell Sci. 2002. PMID: 12045218 - Microtubule dynamics differentially regulates Rho and Rac activity and triggers Rho-independent stress fiber formation in macrophage polykaryons.
Ory S, Destaing O, Jurdic P. Ory S, et al. Eur J Cell Biol. 2002 Jun;81(6):351-62. doi: 10.1078/0171-9335-00255. Eur J Cell Biol. 2002. PMID: 12113476 - Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices.
Davis GE, Bayless KJ, Mavila A. Davis GE, et al. Anat Rec. 2002 Nov 1;268(3):252-75. doi: 10.1002/ar.10159. Anat Rec. 2002. PMID: 12382323 Review. - What tangled webs they weave: Rho-GTPase control of angiogenesis.
Bryan BA, D'Amore PA. Bryan BA, et al. Cell Mol Life Sci. 2007 Aug;64(16):2053-65. doi: 10.1007/s00018-007-7008-z. Cell Mol Life Sci. 2007. PMID: 17530172 Free PMC article. Review.
Cited by
- Tips, stalks, tubes: notch-mediated cell fate determination and mechanisms of tubulogenesis during angiogenesis.
Tung JJ, Tattersall IW, Kitajewski J. Tung JJ, et al. Cold Spring Harb Perspect Med. 2012 Feb;2(2):a006601. doi: 10.1101/cshperspect.a006601. Cold Spring Harb Perspect Med. 2012. PMID: 22355796 Free PMC article. Review. - Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension.
Johnson JA, Hemnes AR, Perrien DS, Schuster M, Robinson LJ, Gladson S, Loibner H, Bai S, Blackwell TR, Tada Y, Harral JW, Talati M, Lane KB, Fagan KA, West J. Johnson JA, et al. Am J Physiol Lung Cell Mol Physiol. 2012 Mar 1;302(5):L474-84. doi: 10.1152/ajplung.00202.2011. Epub 2011 Dec 16. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22180660 Free PMC article. - Angiogenic Transformation in Human Brain Micro Endothelial Cells: Whole Genome DNA Methylation and Transcriptomic Analysis.
Goyal D, Goyal R. Goyal D, et al. Front Physiol. 2019 Dec 11;10:1502. doi: 10.3389/fphys.2019.01502. eCollection 2019. Front Physiol. 2019. PMID: 31920707 Free PMC article. - Uncovering the behaviors of individual cells within a multicellular microvascular community.
Parsa H, Upadhyay R, Sia SK. Parsa H, et al. Proc Natl Acad Sci U S A. 2011 Mar 22;108(12):5133-8. doi: 10.1073/pnas.1007508108. Epub 2011 Mar 7. Proc Natl Acad Sci U S A. 2011. PMID: 21383144 Free PMC article. - Repression of choroidal neovascularization through actin cytoskeleton pathways by microRNA-24.
Zhou Q, Anderson C, Zhang H, Li X, Inglis F, Jayagopal A, Wang S. Zhou Q, et al. Mol Ther. 2014 Feb;22(2):378-389. doi: 10.1038/mt.2013.243. Epub 2013 Oct 17. Mol Ther. 2014. PMID: 24297048 Free PMC article.
References
- Aktories K, Schmidt G, Just I. Rho GTPases as targets of bacterial protein toxins. Biol Chem. 2000;381:421–426. - PubMed
- Ambler CA, Nowicki JL, Burke AC, Bautch VL. Assembly of trunk and limb blood vessels involves extensive migration and vasculogenesis of somite-derived angioblasts. Dev Biol. 2001;234:352–364. - PubMed
- Bach TL, Barsigian C, Chalupowicz DG, Busler D, Yaen CH, Grant DS, Martinez J. VE-cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels. Exp Cell Res. 1998;238:324–334. - PubMed
- Bazzoni G, Dejana E, Lampugnani MG. Endothelial adhesion molecules in the development of the vascular tree: the garden of forking paths. Curr Opin Cell Biol. 1999;11:573–581. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous