Differential localization in cells of myosin II heavy chain kinases during cytokinesis and polarized migration - PubMed (original) (raw)
Differential localization in cells of myosin II heavy chain kinases during cytokinesis and polarized migration
Wenchuan Liang et al. BMC Cell Biol. 2002.
Abstract
Background: Cortical myosin-II filaments in Dictyostelium discoideum display enrichment in the posterior of the cell during cell migration, and in the cleavage furrow during cytokinesis. Filament assembly in turn is regulated by phosphorylation in the tail region of the myosin heavy chain (MHC). Early studies have revealed one enzyme, MHCK-A, which participates in filament assembly control, and two other structurally related enzymes, MHCK-B and -C. In this report we evaluate the biochemical properties of MHCK-C, and using fluorescence microscopy in living cells we examine the localization of GFP-labeled MHCK-A, -B, and -C in relation to GFP-myosin-II localization.
Results: Biochemical analysis indicates that MHCK-C can phosphorylate MHC with concomitant disassembly of myosin II filaments. In living cells, GFP-MHCK-A displayed frequent enrichment in the anterior of polarized migrating cells, and in the polar region but not the furrow during cytokinesis. GFP-MHCK-B generally displayed a homogeneous distribution. In migrating cells GFP-MHCK-C displayed posterior enrichment similar to that of myosin II, but did not localize with myosin II to the furrow during the early stage of cytokinesis. At the late stage of cytokinesis, GFP-MHCK-C became strongly enriched in the cleavage furrow, remaining there through completion of division.
Conclusion: MHCK-A, -B, and -C display distinct cellular localization patterns suggesting different cellular functions and regulation for each MHCK isoform. The strong localization of MHCK-C to the cleavage furrow in the late stages of cell division may reflect a mechanism by which the cell regulates the progressive removal of myosin II as furrowing progresses.
Figures
Figure 1
Domain organization of Dictyostelium MHCKs. All three enzymes contain a strongly conserved seven-fold WD repeat domain at the carboxyl-terminus. MHCK-A has a unique amino-terminal domain of ~ 500 residues that forms a coiled-coil domain responsible for oligomerization and for localization to anterior actin-rich cell extensions. MHCK-B has an amino-terminal segment of ~ 115 residues of currently unknown function. GFP was fused at the amino-terminus of each MHCK for the studies presented here (at codon 2 in each case). "CAT" indicates position of the conserved protein kinase catalytic domain in each enzyme. "SNPQ" (black boxes) indicates position of segments of MHCK-B and MHCK-C that display low amino acid complexity and are rich in serine, asparagine, proline, and glutamine residues.
Figure 2
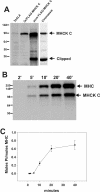
Purification and activity of epitope-tagged MHCK-C. A. MHCK-C expression levels are indicated by western blot analysis of total cell lysates of the 3xALA parental cell line (3XALA lane) and lysates of 3xALA cell overexpressing FLAG-MHCK-C (3x/FLAG-MHCK-C lane). Immunoreactivity of purified FLAG-MHCK-C indicates presence of full length and clipped FLAG-MHCK-C (pure FLAG-MHCK-C lane). Coomassie blue stained material (Coomassie lane) indicates purity and the presence of a clipped breakdown catalytic domain fragment migrating at ~ 35 kDa. Western blot performed with polyclonal antisera generated against the catalytic domain of MHCK-C. B. FLAG-MHCK-C both autophosphorylates and phosphorylates Dictyostelium myosin II on the heavy chain. C. Kinetics and stoichiometry of myosin heavy chain (MHC) phosphorylation by FLAG-MHCK-C. For panels B and C phosphorylation was performed in a reaction mixture consisting of 500 nM MHC (in the form of native myosin II), 100 nM FLAG-MHCK-C, 0.5 mM ATP, 2 mM MgCl2, and 20 mM TES pH 7.0. Error bars represent S.E.M., n = 3
Figure 3
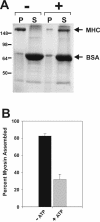
Phosphorylation of myosin II by FLAG-MHCK-C drives filament disassembly. Myosin II was subjected to phosphorylation by FLAG-MHCK-C as for experiments in figure 2. A. Samples containing myosin II (500 nM MHC concentration), FLAG-MHCK-C (100 nM), and BSA (1 μg/μl) were incubated either without ATP (-) or with ATP (+) for 30 minutes, adjusted to 50 mM NaCl for optimal myosin II filament assembly, then subjected to sedimentation at 90,000 ×g for 10 min to pellet assembled filaments. Equal fractions of pellets (P) and supernatants (S) were subjected to SDS-PAGE and Coomassie blue stain. Disassembly is reflected as a loss of MHC in the pellet fractions. No disassembly of myosin occurs if ATP is added in the absence of FLAG-MHCK-C (not shown). B. Densitometric quantification of the percent myosin II in the pellet fractions. Error bars represent S.E.M., n = 5.
Figure 4
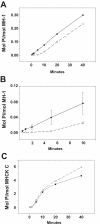
FLAG-MHCK-C is activated by autophosphorylation. The peptide substrate MH-1 is a 16 residue peptide that corresponds to the mapped myosin II phosphorylation site at position 2029 of MHC. A. Phosphorylation of MH-1 by FLAG-MHCK-C occurs with a reproducible lag phase (open symbols), similar to the lag seen with myosin II as the substrate. Preincubation of FLAG-MHCK-C with MgATP eliminates the lag phase (closed symbols). Phosphorylation is plotted in terms of moles Pi transferred as fraction of total moles MH-1 peptide in reaction. B. Expanded plot of early time points of the same experiment. Bars represent S.E.M., n = 3. C. Addition of myosin II to FLAG-MHCK-C autophosphorylation reactions does not accelerate MHCK-C autophosphorylation.
Figure 5
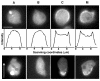
Comparison of interphase D. discoideum cells. GFP-MHCK-A (A), GFP-MHCK-B (B), and GFP-MHCK-C (C) are expressed in Ax2 cells. GFP-myosin II (M) is expressed in myosin II null cells. Scale bar equals to 5 μm. Quantification of the increased accumulation of GFP-proteins in the cortex is obtained by line-scans of the fluorescent intensity profiles across the center of cells (middle row). The x-axis is the scanning coordinate in a unit of μm, and the y-axis is the fluorescence intensities in an arbitrary unit. Interphase cells moving in the upward direction show that GFP-MHCK-A localizes transiently to the anterior pseudopod (A, bottom), while GFP-MHCK-C and GFP-myosin II stay in the posterior region of the cells (C and M, bottom, respectively). GFP-MHCK-B, on the other hand, is homogeneously cytosolic (B, bottom). The brighter cells expressing any of these GFP constructs sometimes display intense fluorescent spots (as in A, top and bottom, and B, bottom) that are likely non-physiological aggregates of the overexpressed protein, as discussed previously [23]. A time-lapse movie in Quicktime format illustrating the anterior localization behavior of MHCK A is available as an additional file (see additional file 1).
Figure 6
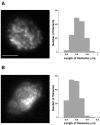
TIRF images of GFP-myosin II (A) and GFP-MHCK-C expressed in the presence of myosin II (B). The fluorescent images show GFP-myosin II thick filaments and GFP-MHCK-C particles in the cortex of a cell attached on a coverslip with a refractive index of 1.78. The distribution of the rod length is displayed next to the images. The mean length of GFP-myosin II and GFP-MHCK-C is 0.6 and 0.3 μm, respectively. The scale bar is 3 μm.
Figure 7
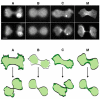
Comparison of GFP-MHCKs and GFP-myosin II distribution during cytokinesis. In the early-to-mid stage of cytokinesis (upper row), none of the GFP-MHCKs localizes to the furrow, opposite to that of the GFP-myosin II (M). GFP-MHCK-A and -C, instead, enriches to the polar protrusions at this stage (A and C, upper row). At the later stage of cytokinesis (lower row), GFP-MHCK-C suddenly appears at the posterior region of the two daughter cells (C), similar to what is observed for GFP-myosin II cells (M). The scale bar shown in the image is 5 μm. The observation described is summarized in the sketch shown below the images.
Figure 8
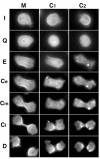
MHCK-C and Myosin II localization at each stage of cytokinesis. Image comparison of cells expressing GFP-MHCK-C (C1 and C2) with GFP-myosin II (M) from the interphase (I), the quiescence (Q), the elongation (E), through the early stage (Ce), the mid-stage (Cm) and the late stage (Cl) of cytokinesis, and finally to the fully divided (D) daughter cells. Although GFP-myosin II localized to the equatorial region early on at the elongation stage and through the whole stages of cytokinesis, GFP-MHCK-C does not appear until the late stage of cytokinesis (Cl). Time lapse movies in Quicktime format corresponding to each series in figure 8 are available as additional files (see additional file 2, additional file 3, and additional file 4).
Figure 9
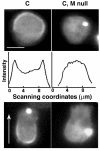
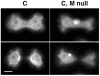
Comparison of GFP-MHCK-C distribution patterns in the interphase of Ax2 (C) and myosin II null (C, M null) cells. In the absence of myosin II, GFP-MHCK-C does not localize to the cell cortex (C, M null, top). A line-scan of the fluorescent intensity profiles across the cells also indicates no cortical distribution in the absence of myosin II (C, M null, middle), the units of x- and y-axis are the same as in Figure 1. In moving cells, direction indicated by arrow, GFP-MHCK-C expressed in the presence of myosin II enriches at the posterior region (C, bottom), GFP-MHCK-C expressed in the myosin II null cells does not stay at the posterior of the cells (C, M null, bottom). The scale bar is 5 μm.
Figure 10
Comparison of GFP-MHCK-C distribution patterns in AX2 (C) and myosin II null (C, M null) cells during cytokinesis. Similar to that expressed in the presence of myosin II, GFP-MHCK-C expressed in the myosin II null cell line does not localize to the furrow at the early stage of cytokinesis (C, M null, upper). However, unlike that expressed in the presence of myosin II, GFP-MHCK-C does not appear at the posterior region of the two leaving daughter cells (C, M null bottom). The scale bar is 5 μm.
Figure 11
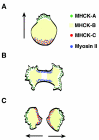
Schematic depiction of differential localization of MHCK-A, -B and -C (in the presence of myosin II) in D. discoideum cells during free migration (A), early stage of cytokinesis (B), and at the completion of cytokinesis (C). In migrating cells, MHCK-C (red dots) colocalizes with myosin II (blue dots) at the posterior region. MHCK-A (green dots), on the other hand, colocalizes with actin at the front protrusions. MHCK-B distributes homogeneously in the cytoplasm (yellow fill). In the early stage of cytokinesis, myosin II concentrates to the furrow. However, MHCK-A (and sometimes MHCK-C) localizes to the polar protrusions (pseudopods) while MHCK-B is always cytosolic throughout the cell with some exclusion from the furrow region. At the late stages of cytokinesis, MHCK-C is recruited to the furrow region, and persists at this location after the completion of division. This persistent localization is reflected as posterior localization in the two new daughter cells, where MHCK-C presumably to help disassemble myosin II thick filaments that have completed their role in furrow contraction.
Similar articles
- Multiple myosin II heavy chain kinases: roles in filament assembly control and proper cytokinesis in Dictyostelium.
Yumura S, Yoshida M, Betapudi V, Licate LS, Iwadate Y, Nagasaki A, Uyeda TQ, Egelhoff TT. Yumura S, et al. Mol Biol Cell. 2005 Sep;16(9):4256-66. doi: 10.1091/mbc.e05-03-0219. Epub 2005 Jun 29. Mol Biol Cell. 2005. PMID: 15987738 Free PMC article. - Novel myosin heavy chain kinase involved in disassembly of myosin II filaments and efficient cleavage in mitotic dictyostelium cells.
Nagasaki A, Itoh G, Yumura S, Uyeda TQ. Nagasaki A, et al. Mol Biol Cell. 2002 Dec;13(12):4333-42. doi: 10.1091/mbc.e02-04-0228. Mol Biol Cell. 2002. PMID: 12475956 Free PMC article. - Actin activation of myosin heavy chain kinase A in Dictyostelium: a biochemical mechanism for the spatial regulation of myosin II filament disassembly.
Egelhoff TT, Croft D, Steimle PA. Egelhoff TT, et al. J Biol Chem. 2005 Jan 28;280(4):2879-87. doi: 10.1074/jbc.M410803200. Epub 2004 Nov 14. J Biol Chem. 2005. PMID: 15545285 - Differential localization of the Dictyostelium kinase DPAKa during cytokinesis and cell migration.
Müller-Taubenberger A, Bretschneider T, Faix J, Konzok A, Simmeth E, Weber I. Müller-Taubenberger A, et al. J Muscle Res Cell Motil. 2002;23(7-8):751-63. doi: 10.1023/a:1024475628061. J Muscle Res Cell Motil. 2002. PMID: 12952073 Review. - Signaling pathways regulating Dictyostelium myosin II.
De la Roche MA, Smith JL, Betapudi V, Egelhoff TT, Côté GP. De la Roche MA, et al. J Muscle Res Cell Motil. 2002;23(7-8):703-18. doi: 10.1023/a:1024467426244. J Muscle Res Cell Motil. 2002. PMID: 12952069 Review.
Cited by
- Cytokinesis mechanics and mechanosensing.
West-Foyle H, Robinson DN. West-Foyle H, et al. Cytoskeleton (Hoboken). 2012 Oct;69(10):700-9. doi: 10.1002/cm.21045. Epub 2012 Jul 3. Cytoskeleton (Hoboken). 2012. PMID: 22761196 Free PMC article. Review. - Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7.
Clark K, Middelbeek J, Morrice NA, Figdor CG, Lasonder E, van Leeuwen FN. Clark K, et al. PLoS One. 2008 Mar 26;3(3):e1876. doi: 10.1371/journal.pone.0001876. PLoS One. 2008. PMID: 18365021 Free PMC article. - A model for cGMP signal transduction in Dictyostelium in perspective of 25 years of cGMP research.
Bosgraaf L, Van Haastert PJ. Bosgraaf L, et al. J Muscle Res Cell Motil. 2002;23(7-8):781-91. doi: 10.1023/a:1024431813040. J Muscle Res Cell Motil. 2002. PMID: 12952076 Review. - Chemotaxis: signalling modules join hands at front and tail.
Postma M, Bosgraaf L, Loovers HM, Van Haastert PJ. Postma M, et al. EMBO Rep. 2004 Jan;5(1):35-40. doi: 10.1038/sj.embor.7400051. EMBO Rep. 2004. PMID: 14710184 Free PMC article. Review. - Myosin II motor proteins with different functions determine the fate of lamellipodia extension during cell spreading.
Betapudi V. Betapudi V. PLoS One. 2010 Jan 5;5(1):e8560. doi: 10.1371/journal.pone.0008560. PLoS One. 2010. PMID: 20052411 Free PMC article.
References
- De Lozanne A, Spudich JA. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987;236:1086–1091. - PubMed
- Knecht DA, Loomis WF. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987;236:1081–1086. - PubMed
- Wessels D, Soll DR, Knecht D, Loomis WF, De Lozanne A, Spudich J. Cell motility and chemotaxis in Dictyostelium amebae lacking myosin heavy chain. Dev Biol. 1988;128:164–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM046551/GM/NIGMS NIH HHS/United States
- R01 GM050009/GM/NIGMS NIH HHS/United States
- 5R01 GM46551-10/GM/NIGMS NIH HHS/United States
- R01 GM50009/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials