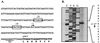Identification, characterization, and regulation of a cluster of genes involved in carbapenem biosynthesis in Photorhabdus luminescens - PubMed (original) (raw)
Identification, characterization, and regulation of a cluster of genes involved in carbapenem biosynthesis in Photorhabdus luminescens
Sylviane Derzelle et al. Appl Environ Microbiol. 2002 Aug.
Abstract
The luminescent entomopathogenic bacterium Photorhabdus luminescens produces several yet-uncharacterized broad-spectrum antibiotics. We report the identification and characterization of a cluster of eight genes (named cpmA to cpmH) responsible for the production of a carbapenem-like antibiotic in strain TT01 of P. luminescens. The cpm cluster differs in several crucial aspects from other car operons. The level of cpm mRNA peaks during exponential phase and is regulated by a Rap/Hor homolog identified in the P. luminescens genome. Marker-exchange mutagenesis of this gene in the entomopathogen decreased antibiotic production. The luxS-like signaling mechanism of quorum sensing also plays a role in the regulation of the cpm operon. Indeed, luxS, which is involved in the production of a newly identified autoinducer, is responsible for repression of cpm gene expression at the end of the exponential growth phase. The importance of this carbapenem production in the ecology of P. luminescens is discussed.
Figures
FIG. 1.
Comparison of the putative P. luminescens cpm operon with the car operon of E. carotovora and Serratia sp. strain ATCC 39006. (A) Map of the genes involved in carbapenem biosynthesis. (B) Amino acid sequence identity between Car and Cpm proteins of E. carotovora, Serratia sp., and P. luminescens.
FIG. 2.
Carbapenem-like activity of P. luminescens visualized by antibiosis. Three-day LB plates spotted with 5 μl of both TT01 (wild type) and mutants were inoculated with 100 μl of various β-lactam indicator strain cultures (OD600 = 0.2) mixed in soft agar. Growth inhibition around a spot indicates production of antibiotics to which the indicator strain is sensitive. All experiments were repeated at least three times. (A) Analysis of carbapenem production and the effect of a cpmA mutation (PL2101) by antibiosis of lawns of various β-lactam indicator strains. (B) Analysis of the effect of various mutations on the ability to produce carbapenem by antibiosis of lawns of Enterobacter cloacae strains C1 (cephalosporinase producer) and C1R (cephalosporinase derepressed). 1, cpmA mutation (PL2101); 2, luxS mutation (PL2102); 3, slyA mutation (PL2103).
FIG. 3.
(A) Nucleotide sequence of the 5′ region of cpmA from P. luminescens. Promoter sequences (−35 and −10 boxes) are boxed. The Shine-Dalgarno sequence (SD) is underlined. Only the first codons of the coding sequence of cpmA are shown in boldface. (B) Primer extension analysis of cpmA transcripts in P. luminescens. Total RNA was extracted from an exponential culture of P. luminescens TT01 grown at 30°C in Schneider medium (OD600 = 1.4). The arrow indicates the position of the extension product obtained.
FIG. 4.
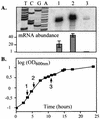
(A) Primer extension analysis of cpm mRNA at various times during P. luminescens growth at 30°C in Schneider medium. Lanes: 1, early log growth (OD600 = 0.3); 2, log growth (OD600 = 1.0); 3, early stationary phase (OD600 = 4.0). Equal amounts of each RNA sample (50 μg) were used. Experiments were done in triplicate. The relative cpm mRNA abundances at different times during P. luminescens growth, with the lowest mRNA level taken as 1, are represented graphically. (B) P. luminescens growth curve indicating the different times when mRNAs were extracted. 1, early log growth (OD600 = 0.3); 2, log growth (OD600 = 1.0); 3, early stationary phase (OD600 = 4.0). Since P. luminescens synthesizes a variety of secondary metabolites which interfere with OD measurement at late exponential phase, correlations between CFU and OD measurements were used to define the various growth stages.
FIG. 5.
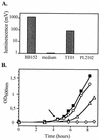
P. luminescens production of a _luxS_-dependent AI-2 activity. (A) AI-2 bioassay of P. luminescens culture supernatants with V. harveyi BB170 as reporter. Ten percent cell-free supernatants were mixed with the reporter strain and incubated at 30°C on a rotary shaker. Light production was determined 4 h 30 min after the BB170 cells were mixed with the various cell-free supernatants (OD600 = 0.1). Cell-free culture fluids were prepared from P. luminescens parental (TT01) and luxS mutant (PL2102) strains grown to an OD600 of 1.0. Schneider sterile medium and V. harveyi BB152 cell-free culture fluid were used as negative and positive controls, respectively. (B) Inhibition of growth by P. luminescens cell-free supernatants. Cell-free supernatants were prepared from a wild-type P. luminescens strain grown in Schneider medium for 5 h (exponential phase; OD600 = 1.0) (open circles), 10 h (early stationary phase; OD600 = 4.0) (open triangles), and 15 h (stationary phase) (open diamonds). Ten percent (vol/vol) cell-free supernatants (open symbols) or sterile Schneider medium (filled squares) was added to the V. harveyi BB170 reporter strain, and cultures were incubated at 30°C on a rotary shaker.
FIG. 6.
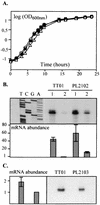
Analysis of the effect of luxS and slyA mutations on growth rate and cpm mRNA synthesis. (A) Growth curve comparison among TT01 (filled squares), PL2102 (luxS mutant) (open triangles), and PL2103 (slyA mutant) (open circles) strains of P. luminescens. (B) Primer extension analysis of the effect of luxS mutation on cpm mRNA synthesis. RNA samples were taken at different times from cultures of TT01 and PL2102. Lanes: 1, log growth (OD600 = 1.0); 2, early stationary phase (OD600 = 4.0). (C) Primer extension analysis of the effect of slyA mutation on cpm mRNA synthesis. RNA samples were taken from exponential-growth-phase cultures of TT01 and PL2103 (OD600 = 0.9). Three independent primer extension analyses were performed. Equal amounts of each RNA sample (50 μg) were used. The relative cpm mRNA abundances measured in TT01 and mutants, with the lowest mRNA level taken as 1, are represented graphically. Error bars represent standard errors of the mean. Statistical analysis was performed with a Student t test, resulting in a significant difference (P < 0.05).
Similar articles
- Cinnamic acid, an autoinducer of its own biosynthesis, is processed via Hca enzymes in Photorhabdus luminescens.
Chalabaev S, Turlin E, Bay S, Ganneau C, Brito-Fravallo E, Charles JF, Danchin A, Biville F. Chalabaev S, et al. Appl Environ Microbiol. 2008 Mar;74(6):1717-25. doi: 10.1128/AEM.02589-07. Epub 2008 Feb 1. Appl Environ Microbiol. 2008. PMID: 18245247 Free PMC article. - Regulatory role of UvrY in adaptation of Photorhabdus luminescens growth inside the insect.
Krin E, Derzelle S, Bedard K, Adib-Conquy M, Turlin E, Lenormand P, Hullo MF, Bonne I, Chakroun N, Lacroix C, Danchin A. Krin E, et al. Environ Microbiol. 2008 May;10(5):1118-34. doi: 10.1111/j.1462-2920.2007.01528.x. Epub 2008 Jan 31. Environ Microbiol. 2008. PMID: 18248456 - Triggering the production of the cryptic blue pigment indigoidine from Photorhabdus luminescens.
Brachmann AO, Kirchner F, Kegler C, Kinski SC, Schmitt I, Bode HB. Brachmann AO, et al. J Biotechnol. 2012 Jan;157(1):96-9. doi: 10.1016/j.jbiotec.2011.10.002. Epub 2011 Nov 4. J Biotechnol. 2012. PMID: 22085970 - Regulation and biosynthesis of carbapenem antibiotics in bacteria.
Coulthurst SJ, Barnard AM, Salmond GP. Coulthurst SJ, et al. Nat Rev Microbiol. 2005 Apr;3(4):295-306. doi: 10.1038/nrmicro1128. Nat Rev Microbiol. 2005. PMID: 15759042 Review. - Toxins and secretion systems of Photorhabdus luminescens.
Rodou A, Ankrah DO, Stathopoulos C. Rodou A, et al. Toxins (Basel). 2010 Jun;2(6):1250-64. doi: 10.3390/toxins2061250. Epub 2010 Jun 1. Toxins (Basel). 2010. PMID: 22069636 Free PMC article. Review.
Cited by
- Exploring Xenorhabdus and Photorhabdus Nematode Symbionts in Search of Novel Therapeutics.
Sajnaga E, Kazimierczak W, Karaś MA, Jach ME. Sajnaga E, et al. Molecules. 2024 Oct 31;29(21):5151. doi: 10.3390/molecules29215151. Molecules. 2024. PMID: 39519791 Free PMC article. Review. - Autoinducer 2 affects biofilm formation by Bacillus cereus.
Auger S, Krin E, Aymerich S, Gohar M. Auger S, et al. Appl Environ Microbiol. 2006 Jan;72(1):937-41. doi: 10.1128/AEM.72.1.937-941.2006. Appl Environ Microbiol. 2006. PMID: 16391139 Free PMC article. - Bacterial autoinduction: looking outside the cell for new metabolic engineering targets.
DeLisa MP, Bentley WE. DeLisa MP, et al. Microb Cell Fact. 2002 Dec 20;1(1):5. doi: 10.1186/1475-2859-1-5. Microb Cell Fact. 2002. PMID: 12537600 Free PMC article. - Cinnamic acid, an autoinducer of its own biosynthesis, is processed via Hca enzymes in Photorhabdus luminescens.
Chalabaev S, Turlin E, Bay S, Ganneau C, Brito-Fravallo E, Charles JF, Danchin A, Biville F. Chalabaev S, et al. Appl Environ Microbiol. 2008 Mar;74(6):1717-25. doi: 10.1128/AEM.02589-07. Epub 2008 Feb 1. Appl Environ Microbiol. 2008. PMID: 18245247 Free PMC article. - The insect-killing bacterium Photorhabdus luminescens has the lowest mutation rate among bacteria.
Pan J, Williams E, Sung W, Lynch M, Long H. Pan J, et al. Mar Life Sci Technol. 2021 Feb;3(1):20-27. doi: 10.1007/s42995-020-00060-0. Epub 2020 Aug 31. Mar Life Sci Technol. 2021. PMID: 33791681 Free PMC article.
References
- Bassler, B. L., M. Wright, and M. R. Silverman. 1993. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9:773-786. - PubMed
- Boemare, N., A. Givaudan, M. Brehelin, and C. Laumond. 1997. Symbiosis and pathogenicity of nematode-bacterium complexes. Symbiosis 22:21-45.
- Bradley, J. S., J. Garau, H. Lode, K. V. Rolston, S. E. Wilson, and J. P. Quinn. 1999. Carbapenems in clinical practice: a guide to their use in serious infection. Int. J. Antimicrob. Agents 11:93-100. - PubMed
- Bycroft, B. W., C. Maslen, S. J. Box, A. Brown, and J. W. Tyler. 1988. The biosynthetic implications of acetate and glutamate incorporation into (3R,5R)-carbapenem-3-carboxylic acid and (5R)-carbapen-2-em-3-carboxylic acid by Serratia sp. J. Antibiot. (Tokyo) 41:1231-1242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources