Reversible memory loss in a mouse transgenic model of Alzheimer's disease - PubMed (original) (raw)
Reversible memory loss in a mouse transgenic model of Alzheimer's disease
Linda A Kotilinek et al. J Neurosci. 2002.
Abstract
Alzheimer's disease (AD) is a neurodegenerative condition, believed to be irreversible, characterized by inexorable deterioration of memory and intellect, with neuronal loss accompanying amyloid plaques and neurofibrillary tangles. In an amyloid precursor protein transgenic mouse model, Tg2576, little or no neuronal loss accompanies age-related memory impairment or the accumulation of Abeta, a 40-42 aa polypeptide found in plaques. Recently, we have shown inverse correlations between brain Abeta and memory in Tg2576 mice stratified by age (Westerman et al., 2002). Broadening the age range examined obscured this relationship, leading us to propose that small, soluble assemblies of Abeta disrupt cognitive function in these mice. Here we show that memory loss can be fully reversed in Tg2576 mice using intraperitoneally administered BAM-10, a monoclonal antibody recognizing the N terminus of Abeta. The beneficial effect of BAM-10 was not associated with a significant Abeta reduction, but instead eliminated the inverse relationship between brain Abeta and memory. We postulate that BAM-10 acts by neutralizing Abeta assemblies in the brain that impair cognitive function. Our results indicate that a substantial portion of memory loss in Tg2576 mice is not permanent. If these Abeta assemblies contribute significantly to memory loss in AD, then successfully targeting them might improve memory in some AD patients.
Figures
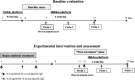
Fig. 1.
Longitudinal experimental design using Tg2576 mice to determine whether memory loss, once present, can be restored. Spatial reference memory was measured, using the Morris water maze (Morris, 1984), immediately before and after intraperitoneal administration of BAM-10, a monoclonal antibody recognizing the N terminus of Aβ.
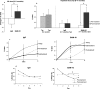
Fig. 2.
Spatial reference learning and memory in 9- to 11-month-old Tg2576 mice before and after treatment with BAM-10 antibody. The change in retention of spatial memory occurring as a result of receiving BAM-10 or IgG antibodies intraperitoneally was measured by subtracting baseline scores from post-treatment scores to obtain the change in percentage of time spent in the target quadrant (Change in %-time).a, Mice 9–11 months of age receiving BAM-10 antibody showed significantly greater improvement than mice receiving nonspecific IgG (*p = 0.03 by _t_test; IgG, n = 17; BAM-10, n = 16). b, In mice that were impaired at baseline (<40% of the time spent in the target quadrant), those receiving BAM-10 antibody also showed significantly greater improvement than those receiving nonspecific IgG (*p = 0.04 by two-way ANOVA with repeated measures; IgG, n = 13; BAM-10,n = 14). Post-treatment performance of impaired mice receiving BAM-10 antibody was significantly higher than baseline performance (*p = 0.01 by paired _t_test) and was similar to that of 2-month-old Tg2576 mice (n = 17) and 3-month-old nontransgenic littermates (n = 10). c, BAM-10, but not nonspecific IgG, restored the retention learning curve of 9- to 11-month-old Tg2576 mice to resemble that of 2-month-old (Young) Tg2576 mice. d, Acquisition of spatial reference memory improved in impaired mice receiving BAM-10 antibody, with significantly reduced mean escape latencies on days 3–5 (*p = 0.04 by paired t test), but not in mice receiving nonspecific IgG. There was a significant treatment-by-training session (baseline vs post-treatment) interaction (p = 0.03 by two-way ANOVA with repeated measures).
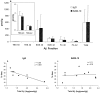
Fig. 3.
Aβ levels in Tg2576 mice treated with BAM-10 or nonspecific IgG antibody. Total Aβ is the sum of Aβ40 and Aβ42 in TBS, 2% SDS, and formic acid (FA) soluble fractions measured as described previously (Kawarabayashi et al., 2001). a, Treatment of mice with BAM-10 was not associated with a significant reduction in total Aβ or in Aβ40 or Aβ42 in any of the fractions analyzed (p values ranged from 0.2 to 0.9). Measurements represent means ± SDs. Brain Aβ levels were correlated with memory in 8.7-month-old Tg2576 mice treated with BAM-10 or nonspecific IgG antibody. b, There was a significant inverse correlation between total Aβ and probe scores in control mice treated with nonspecific IgG.c, Treatment with BAM-10 eliminated the correlation between total Aβ and probe scores.
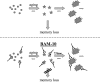
Fig. 4.
BAM-10 neutralizes the cognitively disruptive activity of small Aβ assemblies in the brain. Top, Memory loss in Tg2576 mice appears to be caused by small Aβ assemblies (stars) (Westerman et al., 2002) formed during the conversion of Aβ monomers (circles) to amyloid deposits (starbursts). Aging refers to the event or series of events occurring as animals age leading to the initial aggregation of monomeric Aβ. Little is known about what comprises these events. Bottom, BAM-10 penetrates into the brain, where it may bind to these small Aβ assemblies, neutralize their deleterious effects on cognitive function, and rapidly restore memory in Tg2576 mice. With prolonged treatment, a reduction in amyloid deposits may occur.
Similar articles
- The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease.
Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. Westerman MA, et al. J Neurosci. 2002 Mar 1;22(5):1858-67. doi: 10.1523/JNEUROSCI.22-05-01858.2002. J Neurosci. 2002. PMID: 11880515 Free PMC article. - Acetylcholinesterase inhibitors ameliorate behavioral deficits in the Tg2576 mouse model of Alzheimer's disease.
Dong H, Csernansky CA, Martin MV, Bertchume A, Vallera D, Csernansky JG. Dong H, et al. Psychopharmacology (Berl). 2005 Aug;181(1):145-52. doi: 10.1007/s00213-005-2230-6. Epub 2005 Oct 15. Psychopharmacology (Berl). 2005. PMID: 15778881 Free PMC article. - Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model.
Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Dodart JC, et al. Nat Neurosci. 2002 May;5(5):452-7. doi: 10.1038/nn842. Nat Neurosci. 2002. PMID: 11941374 - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review. - Intracerebroventricular passive immunization in transgenic mouse models of Alzheimer's disease.
Chauhan NB, Siegel GJ. Chauhan NB, et al. Expert Rev Vaccines. 2004 Dec;3(6):717-25. doi: 10.1586/14760584.3.6.717. Expert Rev Vaccines. 2004. PMID: 15606357 Review.
Cited by
- Immunolocalization of Kisspeptin Associated with Amyloid-β Deposits in the Pons of an Alzheimer's Disease Patient.
Chilumuri A, Ashioti M, Nercessian AN, Milton NG. Chilumuri A, et al. J Neurodegener Dis. 2013;2013:879710. doi: 10.1155/2013/879710. Epub 2013 May 16. J Neurodegener Dis. 2013. PMID: 26317001 Free PMC article. - Anti-amyloid-beta immunotherapy in Alzheimer's disease: relevance of transgenic mouse studies to clinical trials.
Wilcock DM, Colton CA. Wilcock DM, et al. J Alzheimers Dis. 2008 Dec;15(4):555-69. doi: 10.3233/jad-2008-15404. J Alzheimers Dis. 2008. PMID: 19096156 Free PMC article. Review. - The regulation of AβPP expression by RNA-binding proteins.
Westmark CJ, Malter JS. Westmark CJ, et al. Ageing Res Rev. 2012 Sep;11(4):450-9. doi: 10.1016/j.arr.2012.03.005. Epub 2012 Apr 5. Ageing Res Rev. 2012. PMID: 22504584 Free PMC article. Review. - Olfactory functions scale with circuit restoration in a rapidly reversible Alzheimer's disease model.
Cheng N, Bai L, Steuer E, Belluscio L. Cheng N, et al. J Neurosci. 2013 Jul 24;33(30):12208-17. doi: 10.1523/JNEUROSCI.0291-13.2013. J Neurosci. 2013. PMID: 23884929 Free PMC article. - Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound.
Jordão JF, Thévenot E, Markham-Coultes K, Scarcelli T, Weng YQ, Xhima K, O'Reilly M, Huang Y, McLaurin J, Hynynen K, Aubert I. Jordão JF, et al. Exp Neurol. 2013 Oct;248:16-29. doi: 10.1016/j.expneurol.2013.05.008. Epub 2013 May 21. Exp Neurol. 2013. PMID: 23707300 Free PMC article.
References
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, Schenk D, Hyman BT. Imaging of amyloid-β deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001;7:369–372. - PubMed
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzehimer's disease model. Nat Neurosci. 2002;5:452–457. - PubMed
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG15453/AG/NIA NIH HHS/United States
- AG08687/AG/NIA NIH HHS/United States
- R01 MH065465/MH/NIMH NIH HHS/United States
- R01 NS033249/NS/NINDS NIH HHS/United States
- MH65465/MH/NIMH NIH HHS/United States
- NS33249/NS/NINDS NIH HHS/United States
- P01 AG015453/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous