Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility - PubMed (original) (raw)
Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility
Vassil D Dontchev et al. J Neurosci. 2002.
Abstract
Neurotrophins and semaphorin 3A are present along pathways and in targets of developing axons of dorsal root ganglion (DRG) sensory neurons. Growth cones of sensory axons are probably regulated by interaction of cytoplasmic signaling triggered coincidentally by both types of guidance molecules. We investigated the in vitro interactions of neurotrophins and semaphorin 3A (Sema3A) in modulating growth cone behaviors of axons extended from DRGs of embryonic day 7 chick embryos. Growth cones of DRGs raised in media containing 10(-9) m NGF or BDNF were more resistant to Sema3A-induced growth cone collapse than when DRGs were raised in 10(-11) m NGF. After overnight culture in 10(-11) m NGF, a 1 hr treatment with 10(-9) m NGF or BDNF was sufficient to increase growth cone resistance to Sema3A-induced collapse. This neurotrophin-mediated decrease in the collapse response of DRG growth cones was not associated with reduced expression on growth cones of the Sema3A-binding protein neuropilin-1. A series of pharmacological studies followed. Phosphatidylinositol 3 kinase activity is not required for these effects of NGF. The effects of inhibitors and activators of protein kinase A (PKA) indicate that PKA activity is involved in NGF modulation of Sema3A-induced growth cone collapse. The effects of inhibitors and activators of PKG indicate that PKG activity is involved in Sema3A-induced growth cone collapse. The effects of inhibitors also indicate that Rho-kinase activity is involved in Sema3A-induced growth cone collapse. These results are consistent with the idea that growth cone responses to an individual guidance cue depend on coincident signaling by other guidance cues and by other regulatory pathways.
Figures
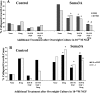
Fig. 1.
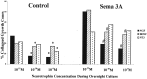
Effects of overnight neurotrophin concentration on growth cone response to Sema3A. DRG explants from E7 embryos were cultured overnight in media containing the neurotrophin concentrations indicated on the _x_-axis. At 24 hr, a predetermined amount of Sema3A was added to half of the dishes for 30 min, followed by fixation with glutaraldehyde. The same volume of solution without Sema3A was added to control dishes before fixation. Each explant was scored for numbers of collapsed and intact growth cones, and the percent collapsed growth cones for each sample population is presented. *p < 0.01, significantly different from 10−11
m
neurotrophin.#p < 0.01, significantly different from 10−11
m
neurotrophin and Sema3A.
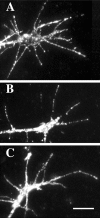
Fig. 2.
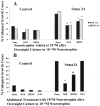
Effects of activation of protein kinase A and elevated [NGF] on growth cone response to Sema3A. DRG explants were cultured overnight in media containing 10−11
m
NGF, and at 24 hr sufficient Sema3A was added to each dish to induce collapse of at least 50% of the DRG growth cones.A, Collapse of a growth cone raised in 10−11
m
NGF and exposed to Sema3A for 30 min. B, Growth cone treated with the PKA activator 8-bromo-cAMP for 135 min before adding Sema3A. Growth cone motility continues after addition of Sema3A. C, Growth cones treated with 10−9
m
NGF for 45 min before adding Sema3A. Two growth cones continue to extend filopodia and advance after addition of Sema3A (arrows). Scale bar, 20 μm.
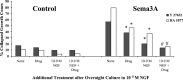
Fig. 3.
Effects of 1 hr culture with 10−9
m
neurotrophin on growth cone response to Sema3A. A, DRG explants were cultured overnight in media containing 10−11
m
NGF or BDNF. At 24 hr, the neurotrophin concentration in some dishes was elevated to 10−9
m
by addition of NGF or BDNF, as indicated. After 1 hr with elevated neurotrophin, Sema3A or an equal volume of control medium was added for 30 min, followed by fixation. The percent collapsed growth cones for each sample population is presented. o/n, Overnight. *p < 0.01, significantly different from 10−11
m
neurotrophin and Sema3A.B, DRG explants were cultured overnight in media containing 10−11
m
NGF. At 24 hr, neurotrophins were added to some dishes to elevate the concentration to 10−9
m
, as indicated. After 1 hr with elevated neurotrophin, Sema3A or an equal volume of control medium was added for 30 min, followed by fixation. The percent collapsed growth cones for each sample population is presented. *p< 0.01, significantly different from Sema3A.#p < 0.01, significantly different from Sema3A and 10−9
m
NGF.
Fig. 4.
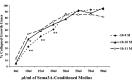
Effect of overnight neurotrophin concentration on growth cone response to increasing concentrations of Sema3A. DRG explants were cultured overnight in media containing the concentrations of NGF indicated in the key. At 24 hr, Sema3A was added for 30 min in the amounts indicated on the _x_-axis, followed by fixation. The percent collapsed growth cones for each sample population is presented. *p < 0.0001, significantly different from 10−11
m
NGF. **p < 0.0001, significantly different from 10−10 and 10−11
m
NGF.
Fig. 5.
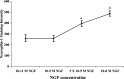
Intensity of anti-neuropilin-1 staining on growth cones cultured in different neurotrophin concentrations. DRG explants were cultured overnight in media containing NGF at the concentrations indicated on the _x_-axis. The explants were fixed and stained with anti-neuropilin-1, and the immunofluorescence staining intensity was determined, as described in Materials and Methods. Data are mean ± SEM for each sample population. *p< 0.01, significantly different from 10−9
m
NGF. #p < 0.001, significantly different from 5 × 10−9
m
NGF. At least 60 growth cones were analyzed at each NGF concentration.
Fig. 6.
Anti-neuropilin-1 staining of growth cones extended from DRGs cultured overnight in 10−9
m
NGF (A), 5 × 10−9
m
NGF (B), and 10−8
m
NGF (C). Fluorescent anti-neuropilin-1 staining is more intense on growth cones raised in 5 × 10−9 or 10−8
m
NGF. Scale bar, 10 μm.
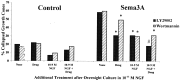
Fig. 7.
Effects of inhibition of PI3 kinase and elevated [NGF] on growth cone response to Sema3A. DRG explants were cultured overnight in media containing 10−11
m
NGF. At 24 hr [NGF] in some dishes was elevated to 10−9
m
for 60 min; 100 n
m
wortmannin or 10 μ
m
LY294002 was added to some dishes for another 60 min; and then Sema3A or control medium was added for 30 min, followed by fixation. The percent collapsed growth cones in each sample population is presented. *p < 0.01, significantly different from Sema3A. #p < 0.01, significantly different from 10−9
m
NGF and Sema3A.
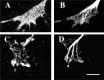
Fig. 8.
Immunocytochemical labeling of DRG growth cones with antibodies against the catalytic unit of PKA (A) and PKG-I (C). These growth cones were also labeled with anti-β-tubulin to label microtubules (B, D). Staining for PKA and PKG is present in the motile peripheral domain of the growth cones. Scale bar, 10 μm.
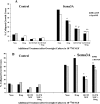
Fig. 9.
A, Effects of PKA activation and elevated [NGF] on growth cone response to Sema3A. DRG explants were cultured overnight in media containing 10−11
m
NGF. At 24 hr, [NGF] in some dishes was elevated to 10−9
m
for 60 min; 2 m
m
8-bromo-cAMP or 2 μ
m
Sp-cAMP was added to some dishes for another 60 min; and then Sema3A or control medium was added for 30 min, followed by fixation. The percent collapsed growth cones for each sample population is presented. The experiments with 8-br-cAMP and Sp-cAMP were conducted at different times, with slightly different levels of collapse in response to Sema3A alone. This accounts for the different heights of bars labeled None. *p < 0.01, significantly different from Sema3A.#p < 0.01, significantly different from drug and Sema3A. **p < 0.01, significantly different from 10−9
m
NGF and Sema3A.B, Effects of PKA inhibition and elevated [NGF] on growth cone response to Sema3A. After overnight culture in media containing 10−11
m
NGF, [NGF] in some dishes was elevated to 10−9
m
for 60 min; 200 n
m
KT5720 or 4 μ
m
PKI was added to some dishes for another 60 min; and then Sema3A or control medium was added for 30 min, followed by fixation. The percent collapsed growth cones in each sample population is presented. *p < 0.01, significantly different from Sema3A. #p < 0.01, significantly different from 10−9
m
NGF and Sema3A.
Fig. 10.
A, Effects of inhibition of protein kinase G and elevated [NGF] on growth cone response to Sema3A. DRG explants were cultured overnight in media containing 10−11
m
NGF. At 24 hr, [NGF] in some dishes was elevated to 10−9
m
for 60 min; 1 μ
m
KT5823 or 100 n
m
ODG was added to some dishes for another 60 min; and then Sema3A or control medium was added for 30 min, followed by fixation. The percent collapsed growth cones in each sample population is presented. The experiments with KT5823 and ODG were conducted at different times, with slightly different levels of collapse in response to Sema3A alone. This accounts for the different heights of bars labeled_None_. *p < 0.01, significantly different from Sema3A. #p < 0.01, significantly different from 10−9
m
NGF and Sema3A. B, Effects of activation of protein kinase G and elevated [NGF] on growth cone response to Sema3A. After overnight culture in media containing 10−11
m
NGF, [NGF] in some dishes was elevated to 10−9
m
for 60 min; 500 μ
m
8-bromo-cGMP or 20 μ
m
YC-1 was added to some dishes for another 60 min; and then Sema3A or control medium was added for 30 min, followed by fixation. The percent collapsed growth cones in each sample population is presented. The experiments with 8-br-cGMP and YC-1 were conducted at different times, with slightly different levels of collapse in response to Sema3A alone. This accounts for the different heights of_bars_ labeled None. *p< 0.01, significantly different from Sema3A.#p < 0.01, significantly different from 10−9
m
NGF and Sema3A.
Fig. 11.
Effects of inhibition of RhoA-activated ROCK kinase and elevated [NGF] on growth cone response to Sema3A. DRG explants were cultured overnight in media containing 10−11
m
NGF. At 24 hr, [NGF] in some dishes was elevated to 10−9
m
for 60 min; 10 μ
m
Y-27632 or 15 μ
m
HA1077 was added to some dishes for another 60 min; and then Sema3A or control medium was added for 30 min, followed by fixation. The percent collapsed growth cones in each sample population is presented. The experiments with Y-27632 and HA1077 were conducted at different times, with slightly different levels of collapse in response to Sema3A alone. This accounts for the different heights of bars labeled_None_. *p < 0.01, significantly different from Sema3A. #p < 0.01, significantly different from 10−9
m
NGF and Sema3A.
Similar articles
- Growth cones integrate signaling from multiple guidance cues.
Dontchev VD, Letourneau PC. Dontchev VD, et al. J Histochem Cytochem. 2003 Apr;51(4):435-44. doi: 10.1177/002215540305100405. J Histochem Cytochem. 2003. PMID: 12642622 Review. - Temporal regulation of neuropilin-1 expression and sensitivity to semaphorin 3A in NGF- and NT3-responsive chick sensory neurons.
Pond A, Roche FK, Letourneau PC. Pond A, et al. J Neurobiol. 2002 Apr;51(1):43-53. doi: 10.1002/neu.10041. J Neurobiol. 2002. PMID: 11920727 - Nerve growth factor, glial cell line-derived neurotrophic factor and neurturin prevent semaphorin 3A-mediated growth cone collapse in adult sensory neurons.
Wanigasekara Y, Keast JR. Wanigasekara Y, et al. Neuroscience. 2006 Oct 13;142(2):369-79. doi: 10.1016/j.neuroscience.2006.06.031. Epub 2006 Jul 28. Neuroscience. 2006. PMID: 16876331 - Semaphorin 3A and neurotrophins: a balance between apoptosis and survival signaling in embryonic DRG neurons.
Ben-Zvi A, Yagil Z, Hagalili Y, Klein H, Lerman O, Behar O. Ben-Zvi A, et al. J Neurochem. 2006 Jan;96(2):585-97. doi: 10.1111/j.1471-4159.2005.03580.x. Epub 2005 Dec 8. J Neurochem. 2006. PMID: 16336628 - Molecular basis of semaphorin-mediated axon guidance.
Nakamura F, Kalb RG, Strittmatter SM. Nakamura F, et al. J Neurobiol. 2000 Aug;44(2):219-29. doi: 10.1002/1097-4695(200008)44:2<219::aid-neu11>3.0.co;2-w. J Neurobiol. 2000. PMID: 10934324 Review.
Cited by
- Micropatterned nanolayers immobilized with nerve growth factor for neurite formation of PC12 cells.
Kim SM, Ueki M, Ren X, Akimoto J, Sakai Y, Ito Y. Kim SM, et al. Int J Nanomedicine. 2019 Sep 19;14:7683-7694. doi: 10.2147/IJN.S217416. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31571871 Free PMC article. - Brain-derived neurotrophic factor attracts geniculate ganglion neurites during embryonic targeting.
Hoshino N, Vatterott P, Egwiekhor A, Rochlin MW. Hoshino N, et al. Dev Neurosci. 2010 Aug;32(3):184-96. doi: 10.1159/000313902. Epub 2010 Jul 20. Dev Neurosci. 2010. PMID: 20639634 Free PMC article. - Vinaxanthone inhibits Semaphorin3A induced axonal growth cone collapse in embryonic neurons but fails to block its growth promoting effects on adult neurons.
Ivakhnitskaia E, Chin MR, Siegel D, Guaiquil VH. Ivakhnitskaia E, et al. Sci Rep. 2021 Jun 21;11(1):13019. doi: 10.1038/s41598-021-92375-w. Sci Rep. 2021. PMID: 34155284 Free PMC article. - Construction of pathways to promote axon growth within the adult central nervous system.
Smith GM, Onifer SM. Smith GM, et al. Brain Res Bull. 2011 Mar 10;84(4-5):300-5. doi: 10.1016/j.brainresbull.2010.05.013. Epub 2010 Jun 8. Brain Res Bull. 2011. PMID: 20554000 Free PMC article. Review. - Modulation of semaphorin3A activity by p75 neurotrophin receptor influences peripheral axon patterning.
Ben-Zvi A, Ben-Gigi L, Klein H, Behar O. Ben-Zvi A, et al. J Neurosci. 2007 Nov 21;27(47):13000-11. doi: 10.1523/JNEUROSCI.3373-07.2007. J Neurosci. 2007. PMID: 18032673 Free PMC article.
References
- Cahoon-Metzger AM, Wang G, Scott SA. Contribution of BDNF-mediated inhibition in patterning avian skin innervation. Dev Biol. 2001;232:246–254. - PubMed
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. - PubMed
- Dickson BJ. Rho GTPases in growth cone guidance. Curr Opin Neurobiol. 2001;11:103–110. - PubMed
- Dong JM, Leung T, Manser E, Lim L. CAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROKinase. J Biol Chem. 1998;273:22554–22562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources