Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF - PubMed (original) (raw)
Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF
George K Tofaris et al. J Neurosci. 2002.
Abstract
Injury to peripheral nerves results in the infiltration of immune cells, which remove axonal- and myelin-derived material. Schwann cells could play a key role in this process by regulating macrophage infiltration. We show here that medium conditioned by primary denervated Schwann cells or the Schwannoma cell line RN22 produces chemotactic activity for macrophages. The presence of blocking antibodies to macrophage chemoattractant protein-1 (MCP-1) or leukemia inhibitory factor (LIF) reduced this activity to approximately 35 and 65% of control levels, respectively, and only 15% remained in the presence of both antibodies. The presence of chemotactic LIF in Schwann cell-conditioned medium was confirmed by using cells from lif-/- mice. Although interleukin-6 (IL-6) is not itself a chemotactic factor, we found that medium from il-6-/- nerves showed only 40% of the activity secreted by wild-type nerves. Furthermore, IL-6 rapidly induced LIF mRNA in primary Schwann cells, and LIF rapidly induced MCP-1 mRNA expression. Treatment of RN22 Schwannoma cells with IL-6 or LIF enhanced the secretion of the chemotactic activity of these cells. These observations show that Schwann cells attract macrophages by secreting MCP-1 and LIF. They also provide evidence for an autocrine-signaling cascade involving IL-6, LIF, and MCP-1, which amplifies the Schwann cell-derived chemotactic signals gradually, in agreement with the delayed entry of macrophages to injured nerves.
Figures
Fig. 1.
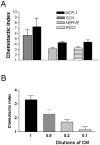
Schwann cells secrete macrophage chemoattractant activity. A, Immunopurified cells (SCH), a Schwann cell line (RN22), and nerve segments (NERVE) secrete macrophage chemoattractant activity. All media were conditioned for 24 hr and used undiluted (see Materials and Methods). For each of these three determinations, MCP-1 (10 ng/ml) served as a positive control in a parallel assay as shown. Defined medium served as negative control. In this and all subsequent illustrations of migration assays, the results are expressed as chemotactic index (see Materials and Methods).B, The Schwann cell-derived macrophage chemotactic activity acts in a dose-dependent manner. Conditioned medium (CM) from immunopurified Schwann cells was used undiluted and at the dilutions indicated.
Fig. 2.
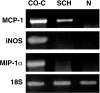
Schwann cells upregulate MCP-1 mRNA when deprived of axonal contact. mRNA levels in freshly isolated nerves (N) are compared with levels in unpurified cultures of dissociated nerve (CO-C) and purified Schwann cells (SCH) after overnight incubation. RT-PCR results are shown for MCP-1-, iNOS-, MIP-1α-, and 18S-specific primers. 18S control samples were used to demonstrate equal loading in all tracks. These results show that purified Schwann cells upregulate MCP-1 mRNA but not MIP-1α or iNOS mRNA when they are deprived of axonal contact in vitro, whereas mixed cultures (containing Schwann cells and fibroblasts) also upregulate iNOS and MIP-1α.
Fig. 3.
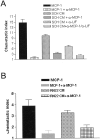
Schwann cells secrete chemotactic activity that is blocked by anti-MCP-1 and/or anti-LIF antibodies. A, Conditioned medium (CM) from primary rat Schwann cells (SCH) contains chemotactic activity that can be blocked by anti-MCP-1 (α-MCP-1) and/or anti-LIF (α-LIF)-neutralizing antibodies. The MCP-1 antibody blocks 60–70% of this activity (p < 0.0001), whereas the LIF antibody blocks 30–40% of this activity (p < 0.006). When the conditioned medium is incubated with both antibodies, only 15% of the chemotactic activity remains (p < 0.0001). B, Neutralizing MCP-1 antibodies reduce chemotactic activity in medium conditioned by RN22 cells by 60% (p < 0.002) in accordance with findings using conditioned medium from primary Schwann cells. A, B, MCP-1 was used as a positive control, and MCP-1 in combination with the anti-MCP-1 antibody was used to confirm the effectiveness of the blocking antibody.
Fig. 4.
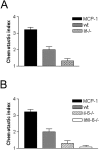
Conditioned medium from nerves of_lif−/−_ mice and il-6−/− mice shows less chemotactic activity than medium from normal wild-type nerves (wt). A, Medium conditioned for 24 hr by nerve segments from lif−/− mice contains significantly less chemotactic activity than media conditioned by nerves from wild-type mice. B, Media conditioned for 24 hr by nerve segments from il-6−/− mice or by nerves from mice lacking both IL-6 and LIF (lif/il-6−/−) contain significantly less chemotactic activity than media from wild-type nerves. The difference between media from il-6−/− and_lif/il-6−/−_ nerves is not statistically significant.A, B, MCP-1 (10 ng/ml) was used as a positive control.
Fig. 5.
IL-6 enhances cytokine expression in Schwann cells. A, IL-6 enhances expression of LIF in purified Schwann cells. The results are from RT-PCR assays of untreated Schwann cells [controls (C)] and Schwann cells treated with 20 ng/ml IL-6 for 6 and 24 hr as indicated. Note that the elevation of LIF mRNA is already clear at 6 hr. 18S control samples were run as shown to control for loading in all tracks. Densitometric comparison of the LIF signals with the corresponding 18S signals shows that LIF elevation is threefold at 6 hr and sevenfold at 24 hr (see Materials and Methods). B, LIF enhances expression of MCP-1 in purified Schwann cells. The results show RT-PCR assays of untreated cells (C) and Schwann cells treated with 20 ng/ml LIF for 1 and 3 hr as indicated. Note that the elevation of MCP-1 mRNA is already clear at 1 hr. 18S control samples were run as shown to control for loading in all tracks. Densitometric comparison of the LIF signals with the corresponding 18S signals shows that MCP-1 elevation is fourfold at 1 hr and fivefold at 3 hr. C, IL-6 enhances expression of MCP-1 in purified Schwann cells. The results show RT-PCR assays of untreated cells (C) and cells treated with 20 ng/ml IL-6 for 6 and 10 hr as indicated. The elevation of MCP-1 mRNA is not unambiguous until the 10 hr point. This delay is consistent with the idea that IL-6 controls MCP-1 levels indirectly by activating LIF (Fig. 7). GAPDH PCR was run as shown to control for loading in all tracks. Densitometric comparison of the LIF signals with the corresponding GAPDH signals shows that MCP-1 elevation is 1.4-fold at 6 hr and threefold at 10 hr.
Fig. 6.
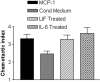
Exogenous LIF or IL-6 induces chemotactic activity in the RN22 Schwann cell line. RN22 cells were treated for 3 hr with 20 ng/ml LIF or IL-6 and then washed extensively. Conditioned medium (Cond Medium) was collected from the cells after an additional 24 hr incubation and tested in the migration assay. LIF and IL-6 increased the level of chemotactic activity in conditioned medium by 34% (p < 0.02) and 44% (p < 0.005), respectively.
Fig. 7.
A tentative model of a cytokine-signaling cascade controlling macrophage (M) entry to damaged nerves. An autocrine cascade of IL-6 and LIF enhances Schwann cell (S) secretion of LIF and MCP-1, both of which directly attract macrophages. This indirect regulation of the major macrophage attractant MCP-1 is in agreement with observed delay in macrophage recruitment to transected nerves in vivo.
Similar articles
- Differential regulation of chemokines by leukemia inhibitory factor, interleukin-6 and oncostatin M.
Hartner A, Goppelt-Struebe M, Hocke GM, Sterzel RB. Hartner A, et al. Kidney Int. 1997 Jun;51(6):1754-60. doi: 10.1038/ki.1997.241. Kidney Int. 1997. PMID: 9186863 - LIF-and IL-1 beta-mediated increases in substance P receptor mRNA in axotomized, explanted or dissociated sympathetic ganglia.
Ludlam WH, Chandross KJ, Kessler JA. Ludlam WH, et al. Brain Res. 1995 Jul 10;685(1-2):12-20. doi: 10.1016/0006-8993(95)00389-8. Brain Res. 1995. PMID: 7583237 - Induction of LIF-mRNA by TGF-beta 1 in Schwann cells.
Matsuoka I, Nakane A, Kurihara K. Matsuoka I, et al. Brain Res. 1997 Nov 21;776(1-2):170-80. doi: 10.1016/s0006-8993(97)01015-9. Brain Res. 1997. PMID: 9439810 - Leukemia inhibitory factor is an autocrine survival factor for Schwann cells.
Dowsing BJ, Morrison WA, Nicola NA, Starkey GP, Bucci T, Kilpatrick TJ. Dowsing BJ, et al. J Neurochem. 1999 Jul;73(1):96-104. doi: 10.1046/j.1471-4159.1999.0730096.x. J Neurochem. 1999. PMID: 10386959 - Leukaemia inhibitory factor is required for normal inflammatory responses to injury in the peripheral and central nervous systems in vivo and is chemotactic for macrophages in vitro.
Sugiura S, Lahav R, Han J, Kou SY, Banner LR, de Pablo F, Patterson PH. Sugiura S, et al. Eur J Neurosci. 2000 Feb;12(2):457-66. doi: 10.1046/j.1460-9568.2000.00922.x. Eur J Neurosci. 2000. PMID: 10712626
Cited by
- Alterations in the expression of leukemia inhibitory factor following exercise: comparisons between wild-type and mdx muscles.
Hunt LC, Anthea Coles C, Gorman CM, Tudor EM, Smythe GM, White JD. Hunt LC, et al. PLoS Curr. 2011 Nov 22;3:RRN1277. doi: 10.1371/currents.RRN1277. PLoS Curr. 2011. PMID: 22183053 Free PMC article. - Mechanisms for Reducing Neuropathic Pain.
Kuffler DP. Kuffler DP. Mol Neurobiol. 2020 Jan;57(1):67-87. doi: 10.1007/s12035-019-01757-9. Epub 2019 Dec 7. Mol Neurobiol. 2020. PMID: 31813127 Review. - Elevated α5 integrin expression on myeloid cells in motor areas in amyotrophic lateral sclerosis is a therapeutic target.
Chiot A, Roemer SF, Ryner L, Bogachuk A, Emberley K, Brownell D, Jimenez GA, Leviten M, Woltjer R, Dickson DW, Steinman L, Ajami B. Chiot A, et al. Proc Natl Acad Sci U S A. 2023 Aug 8;120(32):e2306731120. doi: 10.1073/pnas.2306731120. Epub 2023 Jul 31. Proc Natl Acad Sci U S A. 2023. PMID: 37523555 Free PMC article. - An in-vitro traumatic model to evaluate the response of myelinated cultures to sustained hydrostatic compression injury.
Frieboes LR, Gupta R. Frieboes LR, et al. J Neurotrauma. 2009 Dec;26(12):2245-56. doi: 10.1089/neu.2009.0973. J Neurotrauma. 2009. PMID: 19645529 Free PMC article. - Myeloid cell-mediated targeting of LIF to dystrophic muscle causes transient increases in muscle fiber lesions by disrupting the recruitment and dispersion of macrophages in muscle.
Flores I, Welc SS, Wehling-Henricks M, Tidball JG. Flores I, et al. Hum Mol Genet. 2021 Dec 27;31(2):189-206. doi: 10.1093/hmg/ddab230. Hum Mol Genet. 2021. PMID: 34392367 Free PMC article.
References
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. - PubMed
- Bergsteinsdottir K, Kingston A, Mirsky R, Jessen KR. Rat Schwann cells produce IL-1. J Neuroimmunol. 1991;34:15–23. - PubMed
- Beuche W, Friede RL. The role of non-resident cells in Wallerian degeneration. J Neurocytol. 1984;13:767–796. - PubMed
- Bignold J. Measurement of chemotaxis of polymorphonuclear leukocytes in vitro. J Immunol Methods. 1988;108:1–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous