Intracellular signalling involved in modulating human endothelial barrier function - PubMed (original) (raw)
Review
Intracellular signalling involved in modulating human endothelial barrier function
Victor W M van Hinsbergh et al. J Anat. 2002 Jun.
Abstract
The endothelium dynamically regulates the extravasation of hormones, macromolecules and other solutes. In pathological conditions, endothelial hyperpermeability can be induced by vasoactive agents, which induce tiny leakage sites between the cells, and by cytokines, in particular vascular endothelial growth factor, which increase the exchange of plasma proteins by vesicles and intracellular pores. It is generally believed that the interaction of actin and non-muscle myosin in the periphery of the endothelial cell, and the destabilization of endothelial junctions, are required for endothelial hyperpermeability induced by vasoactive agents. Transient short-term hyperpermeability induced by histamine involves Ca2+/calmodulin-dependent activation of the myosin light chain (MLC) kinase. Prolonged elevated permeability induced by thrombin in addition involves activation of the small GTPase RhoA and Rho kinase, which inhibits dephosphorylation of MLC. It also involves the action of other protein kinases. Several mechanisms can increase endothelial barrier function, depending on the tissue affected and the cause of hyperpermeability. They include blockage of specific receptors, and elevation of cyclic AMP by agents such as beta2-adrenergic agents. Depending on the vascular bed, nitric oxide and cyclic GMP can counteract or aggravate endothelial hyperpermeability. Finally, inhibitors of RhoA activation and Rho kinase represent a potentially valuable group of agents with endothelial hyperpermeability-reducing properties.
Figures
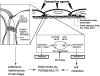
Fig. 1
Endothelial permeability is regulated by MLC phosphorylation and reorganization of the actin cytoskeleton and by tethering forces in the intercellular junctions of endothelial cells. Actin non-muscle–myosin interaction is regulated by myosin light chain (MLC) phosphorylation, which is controlled by Ca2+/calmodulin-dependent activation of MLC kinase (MLCK) and regulation of the MLC phosphatase activity. Both tight junctions (containing occludin, claudins, ZO-1, ZO-2 and ZO-3) and adherens junctions (V,E-cadherin connected via α- and β-catenins and plakoglobin connected to the actin cytoskeleton) contribute to junctional stability. (Adapted from van Hinsbergh, 1997.)
Fig. 2
Histamine increases endothelial permeability and decreases the transendothelial electrical resistance (TEER). The decrease in TEER is inhibited by the Ca2+ chelator BAPTA, the calmodulin inhibitor trifluoperazine (TFP), and the MLCK inhibitor ML-7 (van Nieuw Amerongen et al. 1998). The right panel gives a schematic representation of the mechanism by which histamine increases MLC phosphorylation and actin-non-muscle–myosin interaction. An additional effect of histamine on junctional stability remains possible.
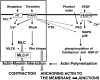
Fig. 3
Schematic representation of the effects of thrombin on the endothelial actin cytoskeleton that contribute to endothelial permeability. The Ca2+/calmodulin-dependent activation of MLCK acts together with the RhoA/Rho kinase-dependent inhibition of MLC phosphatase (Ca2+ sensitization). Thrombin can also activate MAP kinase and protein kinase C (PKC), thereby influencing actin polymerization, and on protein tyrosine kinase (PTK) activity, which may affect junctional integrity.
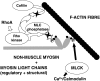
Fig. 4
Schematic picture of the effects of Ca2+/calmodulin-dependent MLCK activity and RhoA/Rho kinase on actin–non–muscle–myosin interaction in endothelial cells. It should be noted that Rho kinase may also have additional effects on the anchoring of the actin cytoskeleton to proteins in the plasma membrane.
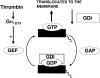
Fig. 5
Schematic representation of the activation of the RhoA. The GTP binding form of RhoA is translocated to the membrane where it is active. Guanine exchange factors (GEFs) activate RhoA, while GTPase-activating proteins (GAPs) bring RhoA back to its inactive form. The guanine dissociation factors (GDIs) dissociate active RhoA from the membrane, facilitate its inactivation, and inhibit its activation by binding to the carboxyl-terminal part of RhoA.
Similar articles
- Targets for pharmacological intervention of endothelial hyperpermeability and barrier function.
van Nieuw Amerongen GP, van Hinsbergh VW. van Nieuw Amerongen GP, et al. Vascul Pharmacol. 2002 Nov;39(4-5):257-72. doi: 10.1016/s1537-1891(03)00014-4. Vascul Pharmacol. 2002. PMID: 12747965 Review. - Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction.
van Nieuw Amerongen GP, Vermeer MA, van Hinsbergh VW. van Nieuw Amerongen GP, et al. Arterioscler Thromb Vasc Biol. 2000 Dec;20(12):E127-33. doi: 10.1161/01.atv.20.12.e127. Arterioscler Thromb Vasc Biol. 2000. PMID: 11116077 - Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability.
Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Sun H, et al. Microcirculation. 2006 Apr-May;13(3):237-47. doi: 10.1080/10739680600556944. Microcirculation. 2006. PMID: 16627366 - Thrombin-induced phosphorylation of the regulatory light chain of myosin II in cultured bovine corneal endothelial cells.
Satpathy M, Gallagher P, Lizotte-Waniewski M, Srinivas SP. Satpathy M, et al. Exp Eye Res. 2004 Oct;79(4):477-86. doi: 10.1016/j.exer.2004.06.018. Exp Eye Res. 2004. PMID: 15381032 - Protein kinase signaling in the modulation of microvascular permeability.
Yuan SY. Yuan SY. Vascul Pharmacol. 2002 Nov;39(4-5):213-23. doi: 10.1016/s1537-1891(03)00010-7. Vascul Pharmacol. 2002. PMID: 12747961 Review.
Cited by
- Role of PI3K/Akt and MEK/ERK Signalling in cAMP/Epac-Mediated Endothelial Barrier Stabilisation.
Gündüz D, Troidl C, Tanislav C, Rohrbach S, Hamm C, Aslam M. Gündüz D, et al. Front Physiol. 2019 Nov 7;10:1387. doi: 10.3389/fphys.2019.01387. eCollection 2019. Front Physiol. 2019. PMID: 31787905 Free PMC article. - Identification and characterization of Dlc1 isoforms in the mouse and study of the biological function of a single gene trapped isoform.
Sabbir MG, Wigle N, Loewen S, Gu Y, Buse C, Hicks GG, Mowat MR. Sabbir MG, et al. BMC Biol. 2010 Mar 3;8:17. doi: 10.1186/1741-7007-8-17. BMC Biol. 2010. PMID: 20199662 Free PMC article. - Perturbation of endothelial junction proteins by Staphylococcus aureus alpha-toxin: inhibition of endothelial gap formation by adrenomedullin.
Hocke AC, Temmesfeld-Wollbrueck B, Schmeck B, Berger K, Frisch EM, Witzenrath M, Brell B, Suttorp N, Hippenstiel S. Hocke AC, et al. Histochem Cell Biol. 2006 Sep;126(3):305-16. doi: 10.1007/s00418-006-0174-5. Epub 2006 Apr 5. Histochem Cell Biol. 2006. PMID: 16596365 - Effect of H1- and H2-histamine receptor blockade on postexercise insulin sensitivity.
Pellinger TK, Dumke BR, Halliwill JR. Pellinger TK, et al. Physiol Rep. 2013 Jul;1(2):e00033. doi: 10.1002/phy2.33. Epub 2013 Jul 18. Physiol Rep. 2013. PMID: 24303118 Free PMC article. - Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age.
EFSA Scientific Committee; Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Bresson JL, Dusemund B, Gundert-Remy U, Kersting M, Lambré C, Penninks A, Tritscher A, Waalkens-Berendsen I, Woutersen R, Arcella D, Court Marques D, Dorne JL, Kass GE, Mortensen A. EFSA Scientific Committee, et al. EFSA J. 2017 May 31;15(5):e04849. doi: 10.2903/j.efsa.2017.4849. eCollection 2017 May. EFSA J. 2017. PMID: 32625502 Free PMC article.
References
- Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am. J. Physiol. 1998;274:H1885–H1894. - PubMed
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. - PubMed
- Andriopoulou P, Navarro P, Zanetti A, Lampugnani MG, Dejana E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler. Thromb. Vasc Biol. 1999;19:2286–2297. - PubMed
- Baluk P, Hirata A, Thurston G, Fujiwara T, Neal CR, Michel CC, et al. Endothelial gaps: time course of formation and closure in inflamed venules of rats. Am. J. Physiol. 1997;272:L155–L170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous