Matrix metalloproteinase-9 deficiency impairs cellular infiltration and bronchial hyperresponsiveness during allergen-induced airway inflammation - PubMed (original) (raw)
Matrix metalloproteinase-9 deficiency impairs cellular infiltration and bronchial hyperresponsiveness during allergen-induced airway inflammation
Didier D Cataldo et al. Am J Pathol. 2002 Aug.
Abstract
We investigated the specific role of matrix metalloproteinase (MMP)-9 in allergic asthma using a murine model of allergen-induced airway inflammation and airway hyperresponsiveness in MMP-9(-/-) mice and their corresponding wild-type (WT) littermates. After a single intraperitoneal sensitization to ovalbumin, the mice were exposed daily either to ovalbumin (1%) or phosphate-buffered saline aerosols from days 14 to 21. Significantly less peribronchial mononuclear cell infiltration of the airways and less lymphocytes in the bronchoalveolar lavage fluid were detected in challenged MMP-9(-/-) as compared to WT mice. In contrast, comparable numbers of bronchoalveolar lavage fluid eosinophils were observed in both genotypes. After allergen exposure, the WT mice developed a significant airway hyperresponsiveness to carbachol whereas the MMP-9(-/-) mice failed to do so. Allergen exposure induced an increase of MMP-9-related gelatinolytic activity in WT lung extracts. Quantitative reverse transcriptase-polymerase chain reaction showed increased mRNA levels of MMP-12, MMP-14, and urokinase-type plasminogen activator after allergen exposure in the lung extracts of WT mice but not in MMP-9-deficient mice. In contrast, the expression of tissue inhibitor of metalloproteinases-1 was enhanced after allergen exposure in both groups. We conclude that MMP-9 plays a key role in the development of airway inflammation after allergen exposure.
Figures
Figure 1.
Allergen-induced pulmonary inflammation. Photomicrographs depicting the pulmonary tissue of the four groups of mice. WT mice exposed to PBS (a) or OVA (b) and MMP-9−/− mice exposed to PBS (c) or OVA (d). H&E: original magnifications, ×200.
Figure 2.
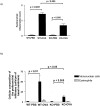
Quantification of histological inflammation. Mean peribronchial inflammation scores (a) and cellular composition of the infiltrates (b) were determined in the four groups as described in the Materials and Methods.
Figure 3.
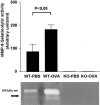
Zymographic analysis of lung proteins extracts. In WT mice, gelatinolytic activity related to MMP-9 protein was significantly increased after allergen exposure. Both pro-MMP-9 (with a molecular weight of 105 kd) and active MMP-9 (arrow) were detected.
Figure 4.
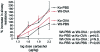
Measurements of airway responsiveness to carbachol. Dose response curves of respiratory system resistance expressed as percent increase from baseline against the intravenous carbachol dose in MMP-9+/+ (WT) and MMP-9−/− mice exposed either to PBS or allergens. In this model, airway resistance is calculated from the differential pressure between the pleural cavity and the airways tidal volume and flow. WT mice exposed to OVA (n = 16) are significantly hyperresponsive compared to WT mice exposed to PBS aerosol (n = 9). In contrast MMP-9−/− mice fail to develop allergen-induced AHR.
Figure 5.
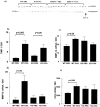
mRNA expression measured by RT-PCR of MMPs, TIMPs, uPA, and PAI. The RNA was extracted from the whole lungs crushed in liquid nitrogen. The results are expressed as a ratio between the intensity of the endogenous band and the band of a synthetic standard RNA and normalized by the same ratio calculated for 28S rRNA. Results are expressed as a number of mRNA copies. a: Representative example of migration on polyacrylamide gels representing TIMP-1 mRNA expression. b–e: The bar graphs represent the ratio 28S rRNA/mRNA and are expressed as mean ± SEM for the gene expression of TIMP-1, MMP-14, MMP-12, and uPA, respectively.
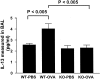
Figure 6.
IL-13 measurements in the BAL. BAL samples were assessed for IL-13 levels using a commercial enzyme-linked immunosorbent assay. The results are expressed as mean ± SEM.
Similar articles
- Inhibition of matrix metalloproteinases prevents allergen-induced airway inflammation in a murine model of asthma.
Kumagai K, Ohno I, Okada S, Ohkawara Y, Suzuki K, Shinya T, Nagase H, Iwata K, Shirato K. Kumagai K, et al. J Immunol. 1999 Apr 1;162(7):4212-9. J Immunol. 1999. PMID: 10201949 - Matrix metalloproteinase-19 deficiency promotes tenascin-C accumulation and allergen-induced airway inflammation.
Gueders MM, Hirst SJ, Quesada-Calvo F, Paulissen G, Hacha J, Gilles C, Gosset P, Louis R, Foidart JM, Lopez-Otin C, Noël A, Cataldo DD. Gueders MM, et al. Am J Respir Cell Mol Biol. 2010 Sep;43(3):286-95. doi: 10.1165/rcmb.2008-0426OC. Epub 2009 Oct 20. Am J Respir Cell Mol Biol. 2010. PMID: 19843707 - Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation.
McMillan SJ, Kearley J, Campbell JD, Zhu XW, Larbi KY, Shipley JM, Senior RM, Nourshargh S, Lloyd CM. McMillan SJ, et al. J Immunol. 2004 Feb 15;172(4):2586-94. doi: 10.4049/jimmunol.172.4.2586. J Immunol. 2004. PMID: 14764732 - Annexin-1-deficient mice exhibit spontaneous airway hyperresponsiveness and exacerbated allergen-specific antibody responses in a mouse model of asthma.
Ng FS, Wong KY, Guan SP, Mustafa FB, Kajiji TS, Bist P, Biswas SK, Wong WS, Lim LH. Ng FS, et al. Clin Exp Allergy. 2011 Dec;41(12):1793-803. doi: 10.1111/j.1365-2222.2011.03855.x. Epub 2011 Sep 20. Clin Exp Allergy. 2011. PMID: 22092555 - Matrix metalloproteinase-9: marker or mediator of tissue damage in asthma?
Shute J. Shute J. Clin Exp Allergy. 2002 Feb;32(2):168-71. doi: 10.1046/j.1365-2222.2002.01302.x. Clin Exp Allergy. 2002. PMID: 11929475 Review. No abstract available.
Cited by
- Impacts of Matrix Metalloproteinase-9 Promoter Genotypes on Asthma Risk.
Chiu KL, He JL, Chen GL, Shen TC, Chen LH, Chen JC, Tsai CW, Chang WS, Hsia TC, Bau DT. Chiu KL, et al. In Vivo. 2024 Sep-Oct;38(5):2144-2151. doi: 10.21873/invivo.13677. In Vivo. 2024. PMID: 39187355 Free PMC article. - Induction of the plasminogen activator system by mechanical stimulation of human bronchial epithelial cells.
Chu EK, Cheng J, Foley JS, Mecham BH, Owen CA, Haley KJ, Mariani TJ, Kohane IS, Tschumperlin DJ, Drazen JM. Chu EK, et al. Am J Respir Cell Mol Biol. 2006 Dec;35(6):628-38. doi: 10.1165/rcmb.2006-0040OC. Epub 2006 Jun 22. Am J Respir Cell Mol Biol. 2006. PMID: 16794260 Free PMC article. - Type-I interferons induce lung protease responses following respiratory syncytial virus infection via RIG-I-like receptors.
Foronjy RF, Taggart CC, Dabo AJ, Weldon S, Cummins N, Geraghty P. Foronjy RF, et al. Mucosal Immunol. 2015 Jan;8(1):161-75. doi: 10.1038/mi.2014.54. Epub 2014 Jul 9. Mucosal Immunol. 2015. PMID: 25005357 Free PMC article. - Matrix metalloproteinases: old dogs with new tricks.
Somerville RP, Oblander SA, Apte SS. Somerville RP, et al. Genome Biol. 2003;4(6):216. doi: 10.1186/gb-2003-4-6-216. Epub 2003 May 29. Genome Biol. 2003. PMID: 12801404 Free PMC article. Review. - MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs.
Sitia G, Isogawa M, Iannacone M, Campbell IL, Chisari FV, Guidotti LG. Sitia G, et al. J Clin Invest. 2004 Apr;113(8):1158-67. doi: 10.1172/JCI21087. J Clin Invest. 2004. PMID: 15085195 Free PMC article.
References
- Wardlaw AJ, Dunnette S, Gleich GJ, Collins JV, Kay AB: Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis 1988, 137:62-69 - PubMed
- Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanovic R: The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med 2000, 161:9-16 - PubMed
- Kips JC, Tournoy KG, Pauwels RA: Gene knockout models of asthma. Am J Respir Crit Care Med 2000, 162:S66-S70 - PubMed
- Nagase H: Activation mechanisms of matrix metalloproteinases. Biol Chem 1997, 378:151-160 - PubMed
- Kjeldsen L, Sengelov H, Lollike K, Nielsen MH, Borregaard N: Isolation and characterization of gelatinase granules from human neutrophils. Blood 1994, 83:1640-1649 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous