Hijacking the translation apparatus by RNA viruses - PubMed (original) (raw)
Review
Hijacking the translation apparatus by RNA viruses
Martin Bushell et al. J Cell Biol. 2002.
Abstract
As invading viruses do not harbor functional ribosomes in their virions, successful amplification of the viral genomes requires that viral mRNAs compete with cellular mRNAs for the host cell translation apparatus. Several RNA viruses have evolved remarkable strategies to recruit the host translation initiation factors required for the first steps in translation initiation by host cell mRNAs. This review describes the ways that three families of RNA viruses effectively usurp limiting translation initiation factors from the host.
Figures
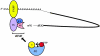
Figure 1.
Model depicting the major participants that are involved in translational initiation in eukaryotic mRNAs. Interactions of eukaryotic translation initiation factors eIF2 (2), eIF3 (3), and initiator tRNA with a 40S ribosomal subunit, eIF4E (4E), eIF4A (4A), and eIF4G (4G) with the m7G cap structure, and the polyadenosine binding protein PABP with the polyadenosine tail in an mRNA are shown.
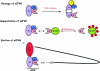
Figure 2.
Alterations of the cap binding protein complex eIF4F in infected cells. (Top) Cleavage of eIF4G by picornaviral proteases. (Middle) Sequestration of eIF4E by 4E binding proteins (4E-BP) due to dephosphorylation of 4E-BPs in picornavirus-infected cells. (Bottom) Eviction of PABP by viral NSP3 from the cap binding protein complex eIF4F in rotavirus- infected cells. Interactions of eukaryotic initiation factors eIF4G (4G), eIF4A (4A), eIF4E (4E), and eIF3 (3) are indicated.
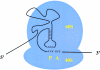
Figure 3.
Occupation of the ribosomal P-site by the cricket paralysis virus IRES. Basepair interactions between sequences in the viral IRES positioned in the ribosomal P-site (P) are diagramed. An empty ribosomal A-site (A) that can accept the first elongator tRNA molecule is shown.
Similar articles
- Translational control by viral proteinases.
Lloyd RE. Lloyd RE. Virus Res. 2006 Jul;119(1):76-88. doi: 10.1016/j.virusres.2005.10.016. Epub 2005 Nov 21. Virus Res. 2006. PMID: 16303201 Free PMC article. Review. - Inhibition of cell functions by RNA-virus infections.
Kääriäinen L, Ranki M. Kääriäinen L, et al. Annu Rev Microbiol. 1984;38:91-109. doi: 10.1146/annurev.mi.38.100184.000515. Annu Rev Microbiol. 1984. PMID: 6093688 Review. - Rotavirus NSP3 Is a Translational Surrogate of the Poly(A) Binding Protein-Poly(A) Complex.
Gratia M, Sarot E, Vende P, Charpilienne A, Baron CH, Duarte M, Pyronnet S, Poncet D. Gratia M, et al. J Virol. 2015 Sep;89(17):8773-82. doi: 10.1128/JVI.01402-15. Epub 2015 Jun 10. J Virol. 2015. PMID: 26063427 Free PMC article. - Viral subversion of the host protein synthesis machinery.
Walsh D, Mohr I. Walsh D, et al. Nat Rev Microbiol. 2011 Oct 17;9(12):860-75. doi: 10.1038/nrmicro2655. Nat Rev Microbiol. 2011. PMID: 22002165 Free PMC article. Review.
Cited by
- Interplay between polyadenylate-binding protein 1 and Kaposi's sarcoma-associated herpesvirus ORF57 in accumulation of polyadenylated nuclear RNA, a viral long noncoding RNA.
Massimelli MJ, Majerciak V, Kruhlak M, Zheng ZM. Massimelli MJ, et al. J Virol. 2013 Jan;87(1):243-56. doi: 10.1128/JVI.01693-12. Epub 2012 Oct 17. J Virol. 2013. PMID: 23077296 Free PMC article. - Nuclear translation visualized by ribosome-bound nascent chain puromycylation.
David A, Dolan BP, Hickman HD, Knowlton JJ, Clavarino G, Pierre P, Bennink JR, Yewdell JW. David A, et al. J Cell Biol. 2012 Apr 2;197(1):45-57. doi: 10.1083/jcb.201112145. J Cell Biol. 2012. PMID: 22472439 Free PMC article. - Stress granules in the viral replication cycle.
Montero H, Trujillo-Alonso V. Montero H, et al. Viruses. 2011 Nov;3(11):2328-2338. doi: 10.3390/v3112328. Epub 2011 Nov 18. Viruses. 2011. PMID: 22163347 Free PMC article. Review. - Early dengue virus protein synthesis induces extensive rearrangement of the endoplasmic reticulum independent of the UPR and SREBP-2 pathway.
Peña J, Harris E. Peña J, et al. PLoS One. 2012;7(6):e38202. doi: 10.1371/journal.pone.0038202. Epub 2012 Jun 4. PLoS One. 2012. PMID: 22675522 Free PMC article. - Translation control by protein kinase R restricts minute virus of mice infection: role in parvovirus oncolysis.
Ventoso I, Berlanga JJ, Almendral JM. Ventoso I, et al. J Virol. 2010 May;84(10):5043-51. doi: 10.1128/JVI.02188-09. Epub 2010 Mar 10. J Virol. 2010. PMID: 20219905 Free PMC article.
References
- Deo, R.C., C.M. Groft, K.R. Rajashankar, and S.K. Burley. 2002. Recognition of the rotavirus mRNA 3′ consensus by an asymmetric NSP3 homodimer. Cell. 108:71–81. - PubMed
- Dever, T.E. 2002. Gene-specific regulation by general translation factors. Cell. 108:545–556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials