The WD repeat protein, Mdv1p, functions as a molecular adaptor by interacting with Dnm1p and Fis1p during mitochondrial fission - PubMed (original) (raw)
The WD repeat protein, Mdv1p, functions as a molecular adaptor by interacting with Dnm1p and Fis1p during mitochondrial fission
Quinton Tieu et al. J Cell Biol. 2002.
Abstract
Yeast mitochondrial fission is a multistep process during which the dynamin-related GTPase, Dnm1p, assembles into punctate structures that associate with the outer mitochondrial membrane and mediate mitochondrial division. Steps in the Dnm1p-dependent process of fission are regulated by the actions of the WD repeat protein, Mdv1p, and the mitochondrial outer membrane protein, Fis1p. Our previous studies suggested a model where Mdv1p functions to regulate fission at a post-Dnm1p assembly step and Fis1p functions at two distinct steps, at an early point, to regulate Dnm1p assembly, and later, together with Mdv1p, to facilitate Dnm1p-dependent mitochondrial fission. To test this model, we have examined the physical and functional relationship between Mdv1p and Fis1p and present genetic, biochemical, and two-hybrid data indicating that a Fis1p-Mdv1p complex is required to regulate mitochondrial fission. To further define the role of Mdv1p in fission, we examined the structural features of Mdv1p required for its interactions with Dnm1p and Fis1p. Data from two-hybrid analyses and GFP-tagged domains of Mdv1p indicate that it contains two functionally distinct domains that enable it to function as a molecular adaptor to regulate sequential interactions between Dnm1p and Fis1p and catalyze a rate-limiting step in mitochondrial fission.
Figures
Figure 1.
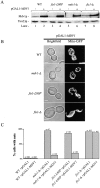
The fis1-L80P mutation disrupts mitochondrial fission, but Dnm1p-containing puncta have wild-type characteristics. Mito-GFP was used to visualize mitochondrial morphology. Dnm1p was visualized using a Dnm1p–GFP fusion protein. (A) Mitochondrial morphology of representative wild-type, gene deletion, and fis1-L80P cells. (B) Dnm1–GFPp localization pattern in fis1-L80P cells. Bars, 2 μm.
Figure 2.
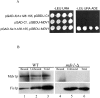
Overexpression of MDV1 suppresses the mitochondrial fission defect in fis1-L80P cells. All strains harboring either the empty pGAL1 vector or pGAL1-MDV1 vectors were grown in SRaf media to early log, subcultured into SGal media, and grown for an additional 12 h and analyzed as described. Mitochondrial morphology was visualized with mito-GFP. (A) Western blot analysis of galactose-induced expression of Mdv1p. (B) Mitochondrial morphology in fis1-L80P cells after overexpression of Mdv1p. C. Quantification of mitochondrial morphology phenotypes in cells overexpressing Mdv1p. Bars, 2 μm.
Figure 3.
Fis1p interacts with Mdv1p by two-hybrid and coimmunoprecipitation analyses. (A) Two-hybrid analysis of MDV1 and FIS1. Interaction between AD fis1_Δ_128–155 and BD MDV1 two-hybrid vectors was assessed by growth of indicated transformants on SD media as described. (B) Immunoprecipitation with anti-Mdv1p antibodies from DSP–cross-linked cells. In vivo, DSP–cross-linked extracts from wild-type and _mdv1-_Δ cells were immunoprecipitated with anti-Mdv1p antibodies and fractions were analyzed by SDS-PAGE and Western blotting as described in the Materials and methods.
Figure 4.
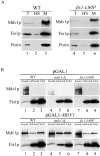
The Fis1p–Mdv1p interaction is disrupted in fis1-L80P cells and restored upon overexpression of Mdv1p. (A) Fractionation of cell extracts from wild-type and fis1-L80P cells by differential centrifugation. Fractions were analyzed by SDS-PAGE and Western blotting as described. (B) In vivo, DSP–cross-linked extracts from cells harboring either pGAL1 or pGAL1-MDV1, grown in SGal media, were immunoprecipitated with anti-Mdv1p antibodies and analyzed by SDS-PAGE and Western blotting.
Figure 5.
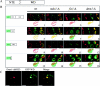
Mdv1p functions as a molecular adaptor during mitochondrial fission. (A) Predicted structural domains of Mdv1p (NTE, C-C, and WD [seven-WD repeat domain]). (B) Dual localization of mitochondria labeled with MitoTracker (in red, left) and GFP-tagged Mdv1p regions (in green, middle) in cells. Overlay (right image) was obtained by merging the signals from MitoTracker and GFP-tagged Mdv1p regions. Indicated strains were grown in SRaf, subcultured in SGal, supplemented with 1% dextrose to partially repress galactose-induced overexpression of Mdv1p domains for optimal visualization, and imaged by deconvolution microscopy. Dextrose was omitted for the cytological analysis of Mdv1p regions in _fis1_-Δ cells because higher expression levels were required to detect interactions with Dnm1p-containing structures. In cells, schematically represented, GFP-tagged Mdv1p regions and mitochondria are depicted in green and red, respectively. (C) GFP–WD and Dnm1–dsRed colocalize in punctate structures in wild-type cells. Cells were grown and analyzed as described in B. Bars, 2 μm.
Similar articles
- The WD40 protein Caf4p is a component of the mitochondrial fission machinery and recruits Dnm1p to mitochondria.
Griffin EE, Graumann J, Chan DC. Griffin EE, et al. J Cell Biol. 2005 Jul 18;170(2):237-48. doi: 10.1083/jcb.200503148. Epub 2005 Jul 11. J Cell Biol. 2005. PMID: 16009724 Free PMC article. - The role of Fis1p-Mdv1p interactions in mitochondrial fission complex assembly.
Karren MA, Coonrod EM, Anderson TK, Shaw JM. Karren MA, et al. J Cell Biol. 2005 Oct 24;171(2):291-301. doi: 10.1083/jcb.200506158. J Cell Biol. 2005. PMID: 16247028 Free PMC article. - Dimeric Dnm1-G385D interacts with Mdv1 on mitochondria and can be stimulated to assemble into fission complexes containing Mdv1 and Fis1.
Bhar D, Karren MA, Babst M, Shaw JM. Bhar D, et al. J Biol Chem. 2006 Jun 23;281(25):17312-17320. doi: 10.1074/jbc.M513530200. Epub 2006 Apr 6. J Biol Chem. 2006. PMID: 16601120 - The machines that divide and fuse mitochondria.
Hoppins S, Lackner L, Nunnari J. Hoppins S, et al. Annu Rev Biochem. 2007;76:751-80. doi: 10.1146/annurev.biochem.76.071905.090048. Annu Rev Biochem. 2007. PMID: 17362197 Review. - The enigmatic role of Mim1 in mitochondrial biogenesis.
Stefan Dimmer K, Rapaport D. Stefan Dimmer K, et al. Eur J Cell Biol. 2010 Feb-Mar;89(2-3):212-5. doi: 10.1016/j.ejcb.2009.11.002. Epub 2009 Nov 26. Eur J Cell Biol. 2010. PMID: 19944477 Review.
Cited by
- Mitochondria-ER-PM contacts regulate mitochondrial division and PI(4)P distribution.
Casler JC, Harper CS, White AJ, Anderson HL, Lackner LL. Casler JC, et al. J Cell Biol. 2024 Sep 2;223(9):e202308144. doi: 10.1083/jcb.202308144. Epub 2024 May 23. J Cell Biol. 2024. PMID: 38781029 - Serine 39 in the GTP-binding domain of Drp1 is involved in shaping mitochondrial morphology.
Ghani M, Szabó B, Alkhatibe M, Amsalu H, Zohar P, Janka EA, Mótyán JA, Tar K. Ghani M, et al. FEBS Open Bio. 2024 Jul;14(7):1147-1165. doi: 10.1002/2211-5463.13820. Epub 2024 May 17. FEBS Open Bio. 2024. PMID: 38760979 Free PMC article. - Stable knockdown of Drp1 improves retinoic acid-BDNF-induced neuronal differentiation through global transcriptomic changes and results in reduced phosphorylation of ERK1/2 independently of DUSP1 and 6.
Ghani M, Zohar P, Ujlaki G, Tóth M, Amsalu H, Póliska S, Tar K. Ghani M, et al. Front Cell Dev Biol. 2024 Mar 14;12:1342741. doi: 10.3389/fcell.2024.1342741. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38550381 Free PMC article. - Biogenesis, inheritance, and 3D ultrastructure of the microsporidian mitosome.
Hacker C, Sendra K, Keisham P, Filipescu T, Lucocq J, Salimi F, Ferguson S, Bhella D, MacNeill SA, Embley M, Lucocq J. Hacker C, et al. Life Sci Alliance. 2023 Oct 30;7(1):e202201635. doi: 10.26508/lsa.202201635. Print 2024 Jan. Life Sci Alliance. 2023. PMID: 37903625 Free PMC article. - The AAA-ATPase Yta4/ATAD1 interacts with the mitochondrial divisome to inhibit mitochondrial fission.
He J, Liu K, Wu Y, Zhao C, Yan S, Chen JH, Hu L, Wang D, Zheng F, Wei W, Xu C, Huang C, Liu X, Yao X, Ding L, Fang Z, Tang AH, Fu C. He J, et al. PLoS Biol. 2023 Aug 17;21(8):e3002247. doi: 10.1371/journal.pbio.3002247. eCollection 2023 Aug. PLoS Biol. 2023. PMID: 37590302 Free PMC article.
References
- Gietz, R.D., and R.H. Schiestl. 1991. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast. 7:253–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases