Consistent patterns in the development and immunodominance of human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T-cell responses following acute HIV-1 infection - PubMed (original) (raw)
Consistent patterns in the development and immunodominance of human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T-cell responses following acute HIV-1 infection
Xu G Yu et al. J Virol. 2002 Sep.
Abstract
Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T-cell responses generated during acute infection play a critical role in the initial control of viremia. However, little is known about the viral T-cell epitopes targeted during acute infection or about their hierarchy in appearance and relative immunodominance over time. In this study, HIV-1-specific CD8+ T-cell responses in 18 acutely infected individuals expressing HLA-A3 and/or -B7 were characterized. Detailed analysis of CD8 responses in one such person who underwent treatment of acute infection followed by reexposure to HIV-1 through supervised treatment interruptions (STI) revealed recognition of only two cytotoxic T-lymphocyte (CTL) epitopes during symptomatic acute infection. HIV-1-specific CD8+ T-cell responses broadened significantly during subsequent exposure to the virus, ultimately targeting 27 distinct CTL epitopes, including 15 different CTL epitopes restricted by a single HLA class I allele (HLA-A3). The same few peptides were consistently targeted in an additional 17 persons expressing HLA-A3 and/or -B7 during acute infection. These studies demonstrate a consistent pattern in the development of epitope-specific responses restricted by a single HLA allele during acute HIV-1 infection, as well as persistence of the initial pattern of immunodominance during subsequent STI. In addition, they demonstrate that HIV-1-specific CD8+ T-cell responses can ultimately target a previously unexpected and unprecedented number of epitopes in a single infected individual, even though these are not detectable during the initial exposure to virus. These studies have important implications for vaccine design and evaluation.
Figures
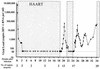
FIG. 1.
Viral loads (HIV-1 RNA copies per ml of plasma) in study subject AC-06 during the 34-month study period are shown on a logarithmic scale. Shading represents periods on HAART. The number of regions targeted by CD8+ T cells in AC-06 is given below the graph.
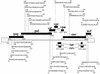
FIG. 2.
HIV-1-specific CD8+ T-cell responses to overlapping peptides in subject AC-06. The amino acid sequences and locations within HIV-1 proteins of all overlapping peptides targeted by virus-specific CD8+ T lymphocytes in subject AC-06 are shown. A total of 25 different regions within HIV-1 were targeted by CD8+ T cells.
FIG. 3.
Characterization of HLA-A3- and HLA-B7-restricted optimal HIV-1-specific CTL epitopes in subject AC-06. Graphs show the HLA class I restriction (left panels) and optimal amino acid sequences (right panels) of two novel HLA-A3-restricted CTL epitopes (A) and two novel HLA-B7-restricted CTL epitopes (B). HLA restriction was determined using peptides presented by autologous and partially HLA matched BCL in a 51Cr release assay. Solid bars, percent specific lysis of target cells pulsed with peptide; hatched bars, percent specific lysis of control target cells pulsed with no peptide. Fine mapping of the optimal epitope was done using serial dilutions of truncated peptides in an IFN-γ ELISPOT assay, and results are given as SFC per 106 PBMC.
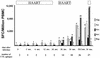
FIG. 4.
Longitudinal evolution of the magnitude and breadth of HIV-1-specific CD8+ T-cell responses on the single-epitope level during treated acute infection and STI in subject AC-06. The magnitudes of CD8+ T-cell responses (given as SFC/106 PBMC) directed against the HIV-1 proteins Gag, Pol, and Env, as well as against the accessory HIV-1 proteins (Vif, Vpr, and Vpu) and the regulatory HIV-1 proteins (Rev and Tat), in subject AC-06 during the 34-month study period are shown. The number of CTL epitopes targeted within each HIV-1 protein or protein group is indicated above each bar. The periods on treatment (HAART) are indicated above the graph; the time after infection and the total number of CTL epitopes targeted at each time point are shown below the graph. Only two CTL epitopes, one in HIV-1 Gag and one in HIV-1 Env, were targeted during acute infection, but virus-specific CD8+ T-cell responses targeted a total of 27 different CTL epitopes at the end of the second STI.
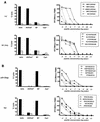
FIG. 5.
Quantification of the contributions of HLA-A3-restricted and HLA-B7- or -Cw7-restricted CD8+ T-cell responses to total HIV-1-specific CD8+ T-cell responses in subject AC-06. PBMC of AC-06 were incubated with autologous BCL pulsed with either the HLA-B7-restricted immunodominant p24 Gag epitope GPGHKARVL (B), all 27 targeted optimal CTL epitopes (C), the 12 HLA-B7- or -Cw7-restricted optimal CTL epitopes (D), or the 15 HLA-A3-restricted optimal CTL epitopes (E) recognized by AC-06. BCL pulsed without peptide were used as negative controls (A). Percentages of peptide-specific IFN-γ-producing CD8+ T cells after subtraction of background activity are given in the individual plots.
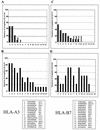
FIG. 6.
Frequency of recognition of optimal CTL epitopes during acute HIV-1 infection and after STI. (A) Percentages of individuals expressing HLA-A3 (n = 14) who recognize the individual HLA-A3-restricted optimal CTL epitopes 1 to 15 during acute HIV-1 infection (solid bars) and after 12 months of treatment with HAART (hatched bars). (B) Percentages of individuals expressing HLA-A3 (n = 7) who recognize the individual HLA-A3-restricted optimal CTL epitopes 1 to 15 following STI. (C) Percentages of individuals expressing HLA-B7 (n = 11) who recognize the individual HLA-B7-restricted optimal CTL epitopes 1 to 15 during acute HIV-1 infection (solid bars) and after 12 months of treatment with HAART (hatched bars). (D) Percentages of individuals expressing HLA-B7 (n = 7) who recognize the individual HLA-B7-restricted optimal CTL epitopes 1 to 15 following STI. The amino acid sequences and corresponding HIV-1 proteins for the CTL epitopes tested are given at the bottom of the figure.
Similar articles
- Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection.
Allen TM, Altfeld M, Yu XG, O'Sullivan KM, Lichterfeld M, Le Gall S, John M, Mothe BR, Lee PK, Kalife ET, Cohen DE, Freedberg KA, Strick DA, Johnston MN, Sette A, Rosenberg ES, Mallal SA, Goulder PJ, Brander C, Walker BD. Allen TM, et al. J Virol. 2004 Jul;78(13):7069-78. doi: 10.1128/JVI.78.13.7069-7078.2004. J Virol. 2004. PMID: 15194783 Free PMC article. - HIV-1-specific cytotoxic T lymphocyte (CTL) responses against immunodominant optimal epitopes slow the progression of AIDS in China.
Zhai S, Zhuang Y, Song Y, Li S, Huang D, Kang W, Li X, Liao Q, Liu Y, Zhao Z, Lu Y, Sun Y. Zhai S, et al. Curr HIV Res. 2008 Jun;6(4):335-50. doi: 10.2174/157016208785132473. Curr HIV Res. 2008. PMID: 18691032 - Relative dominance of epitope-specific cytotoxic T-lymphocyte responses in human immunodeficiency virus type 1-infected persons with shared HLA alleles.
Day CL, Shea AK, Altfeld MA, Olson DP, Buchbinder SP, Hecht FM, Rosenberg ES, Walker BD, Kalams SA. Day CL, et al. J Virol. 2001 Jul;75(14):6279-91. doi: 10.1128/JVI.75.14.6279-6291.2001. J Virol. 2001. PMID: 11413294 Free PMC article. - Immunodominance of HIV-1-specific CD8(+) T-cell responses in acute HIV-1 infection: at the crossroads of viral and host genetics.
Lichterfeld M, Yu XG, Le Gall S, Altfeld M. Lichterfeld M, et al. Trends Immunol. 2005 Mar;26(3):166-71. doi: 10.1016/j.it.2005.01.003. Trends Immunol. 2005. PMID: 15745859 Review. - Characteristics of the intrahepatic cytotoxic T lymphocyte response in chronic hepatitis C virus infection.
Koziel MJ, Walker BD. Koziel MJ, et al. Springer Semin Immunopathol. 1997;19(1):69-83. doi: 10.1007/BF00945026. Springer Semin Immunopathol. 1997. PMID: 9266632 Review.
Cited by
- HIV-1 p24(gag) derived conserved element DNA vaccine increases the breadth of immune response in mice.
Kulkarni V, Rosati M, Valentin A, Ganneru B, Singh AK, Yan J, Rolland M, Alicea C, Beach RK, Zhang GM, Le Gall S, Broderick KE, Sardesai NY, Heckerman D, Mothe B, Brander C, Weiner DB, Mullins JI, Pavlakis GN, Felber BK. Kulkarni V, et al. PLoS One. 2013;8(3):e60245. doi: 10.1371/journal.pone.0060245. Epub 2013 Mar 28. PLoS One. 2013. PMID: 23555935 Free PMC article. - Constraints on HIV-1 evolution and immunodominance revealed in monozygotic adult twins infected with the same virus.
Draenert R, Allen TM, Liu Y, Wrin T, Chappey C, Verrill CL, Sirera G, Eldridge RL, Lahaie MP, Ruiz L, Clotet B, Petropoulos CJ, Walker BD, Martinez-Picado J. Draenert R, et al. J Exp Med. 2006 Mar 20;203(3):529-39. doi: 10.1084/jem.20052116. Epub 2006 Mar 13. J Exp Med. 2006. PMID: 16533886 Free PMC article. - Identification of cryptic MHC I-restricted epitopes encoded by HIV-1 alternative reading frames.
Cardinaud S, Moris A, Février M, Rohrlich PS, Weiss L, Langlade-Demoyen P, Lemonnier FA, Schwartz O, Habel A. Cardinaud S, et al. J Exp Med. 2004 Apr 19;199(8):1053-63. doi: 10.1084/jem.20031869. Epub 2004 Apr 12. J Exp Med. 2004. PMID: 15078897 Free PMC article. - Enhanced detection of human immunodeficiency virus type 1-specific T-cell responses to highly variable regions by using peptides based on autologous virus sequences.
Altfeld M, Addo MM, Shankarappa R, Lee PK, Allen TM, Yu XG, Rathod A, Harlow J, O'Sullivan K, Johnston MN, Goulder PJ, Mullins JI, Rosenberg ES, Brander C, Korber B, Walker BD. Altfeld M, et al. J Virol. 2003 Jul;77(13):7330-40. doi: 10.1128/jvi.77.13.7330-7340.2003. J Virol. 2003. PMID: 12805431 Free PMC article. - TCR affinity associated with functional differences between dominant and subdominant SIV epitope-specific CD8+ T cells in Mamu-A*01+ rhesus monkeys.
Osuna CE, Gonzalez AM, Chang HH, Hung AS, Ehlinger E, Anasti K, Alam SM, Letvin NL. Osuna CE, et al. PLoS Pathog. 2014 Apr 17;10(4):e1004069. doi: 10.1371/journal.ppat.1004069. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24743648 Free PMC article.
References
- Allen, T. M., B. R. Mothe, J. Sidney, P. Jing, J. L. Dzuris, M. E. Liebl, T. U. Vogel, D. H. O'Connor, X. Wang, M. C. Wussow, J. A. Thomson, J. D. Altman, D. I. Watkins, and A. Sette. 2001. CD8+ lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule Mamu-A∗01: implications for vaccine design and testing. J. Virol. 75:738-749. - PMC - PubMed
- Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. - PubMed
- Altfeld, M., M. M. Addo, R. L. Eldridge, X. G. Yu, S. Thomas, A. Khatri, D. Strick, M. N. Phillips, G. B. Cohen, S. A. Islam, S. A. Kalams, C. Brander, P. J. R. Goulder, and B. D. Walker. 2001. Vpr is preferentially targeted by cytotoxic T lymphocytes during HIV-1 infection. J. Immunol. 167:2743-2752.. - PubMed
- Altfeld, M., and E. S. Rosenberg. 2000. The role of CD4+ T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr. Opin. Immunol. 12:375-380. - PubMed
- Altfeld, M., E. S. Rosenberg, R. Shankarappa, J. S. Mukherjee, F. M. Hecht, R. L. Eldridge, M. M. Addo, S. H. Poon, M. N. Phillips, G. K. Robbins, P. E. Sax, S. Boswell, J. O. Kahn, C. Brander, P. J. Goulder, J. A. Levy, J. I. Mullins, and B. D. Walker. 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193:169-180. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI040873/AI/NIAID NIH HHS/United States
- R01 AI44656/AI/NIAID NIH HHS/United States
- R01 AI30914/AI/NIAID NIH HHS/United States
- R01 AI50429/AI/NIAID NIH HHS/United States
- R37 AI125568/AI/NIAID NIH HHS/United States
- R01 AI40873/AI/NIAID NIH HHS/United States
- R01 AI050429/AI/NIAID NIH HHS/United States
- R01 AI044656/AI/NIAID NIH HHS/United States
- R01 AI030914/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials