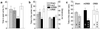CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli - PubMed (original) (raw)
. 2002 Aug 20;99(17):11435-40.
doi: 10.1073/pnas.172091899. Epub 2002 Aug 6.
Jocelien D A Olivier, Linda I Perrotti, Ralph J DiLeone, Olivier Berton, Amelia J Eisch, Soren Impey, Daniel R Storm, Rachael L Neve, Jerry C Yin, Venetia Zachariou, Eric J Nestler
Affiliations
- PMID: 12165570
- PMCID: PMC123274
- DOI: 10.1073/pnas.172091899
CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli
Michel Barrot et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The transcription factor cAMP response element (CRE)-binding protein (CREB) has been shown to regulate neural plasticity. Drugs of abuse activate CREB in the nucleus accumbens, an important part of the brain's reward pathways, and local manipulations of CREB activity have been shown to affect cocaine reward, suggesting an active role of CREB in adaptive processes that follow exposure to drugs of abuse. Using CRE-LacZ reporter mice, we show that not only rewarding stimuli such as morphine, but also aversive stimuli such as stress, activate CRE-mediated transcription in the nucleus accumbens shell. Using viral-mediated gene transfer to locally alter the activity of CREB, we show that this manipulation affects morphine reward, as well as the preference for sucrose, a more natural reward. We then show that local changes in CREB activity induce a more general syndrome, by altering reactions to anxiogenic, aversive, and nociceptive stimuli as well. Increased CREB activity in the nucleus accumbens shell decreases an animal's responses to each of these stimuli, whereas decreased CREB activity induces an opposite phenotype. These results show that environmental stimuli regulate CRE-mediated transcription within the nucleus accumbens shell, and that changes in CREB activity within this brain area subsequently alter gating between emotional stimuli and their behavioral responses. This control appears to be independent of the intrinsic appetitive or aversive value of the stimulus. The potential relevance of these data to addiction and mood disorders is discussed.
Figures
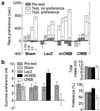
Fig 1.
Response to rewarding stimuli. (a) Morphine place preference after sham surgery (n = 10–18), or expression of LacZ (n = 7–10), mCREB (n = 5–11), or CREB (n = 6–13). Four morphine doses were tested (indicated in mg/ml under the Sham bar graph). CREB overexpression reduced the sensitivity to morphine, whereas mCREB expression increased it. (b) Sucrose preference. Sham surgery (n = 14) or β-gal expression (n = 15) did not affect the sucrose preference; CREB overexpression (n = 16) reduced it, whereas mCREB expression (n = 12) increased it. Data are presented as difference in liquid intake between the two bottles (sucrose vs. water), or as sucrose intake in percentage of the total fluid intake (Lower Right). CREB manipulation did not affect the total fluid intake (Upper Right).
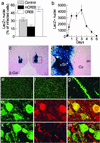
Fig 2.
Viral-mediated gene transfer. (a) In CRE-LacZ reporter mice, the proportion of infected cells expressing β-gal is increased with CREB overexpression (n = 5) and decreased by mCREB expression (n = 5), as compared with cells infected by a control virus expressing a GFP fusion protein (n = 4). (b) Time course of transgene expression determined in rats by using HSV-LacZ (n = 5–8 accumbens). (c) Bilateral injection of HSV-LacZ in the nucleus accumbens shell revealed by X-Gal assay. (d) Higher magnification showing that the infection is restricted to the shell and does not diffuse to the core (ac, anterior commissure; Co, core; Sh, shell). (e and f) CREB immunoreactivity in the nucleus accumbens shell in a control (e) or in an HSV-CREB (f) injected side. (g_–_m) The majority of the infected cells are medium spiny neurons, as shown by β-gal positive processes of HSV-LacZ infected cells (g), and by the colocalization of β-gal (h) with DARPP-32 (i) (merged confocal image in j). No colocalization of β-gal (k) and GFAP (l) was observed (merged confocal image in m).
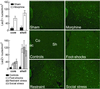
Fig 3.
CRE-mediated transcription. In CRE-LacZ mice, chronic morphine (n = 3, Top Right) increases the density of β-gal positive neurons in the nucleus accumbens as compared with sham-operated mice (n = 4, Top Left). Foot shocks (n = 3, Middle Right), restraint stress (n = 3, Bottom Left), and social stress (n = 3, Bottom Right) also increase CRE-mediated transcription as compared with controls (n = 5, Middle Left). ac, anterior commissure; Co, core; Sh, shell.
Fig 4.
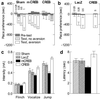
Anxiety-related behaviors. (a) In the elevated plus-maze, local mCREB expression reduces the time spent in the open-arms (n = 7–8). (b) In the open-field (250 lx), similar results were obtained with the time spent in the center of the test (Left; n = 8–12). No influence of CREB was observed on the locomotor activity of the same rats (Right). (c) Comparison between the 250-lx illumination and a less anxiogenic condition (3 lx; n = 6–9) shows that higher illumination reduces the time spent in the center of the test. This anxiogenic effect of light intensity disappears with CREB overexpression, and is enhanced with mCREB expression.
Fig 5.
Response to aversive and nociceptive stimuli. (a) Naloxone place aversion after sham surgery (n = 12–18), or expression of mCREB (n = 6–10) or CREB (n = 5–8) in the nucleus accumbens shell. Four naloxone doses were tested (indicated in mg/ml above the Sham bar graph). CREB overexpression reduced naloxone aversion, whereas mCREB expression increased it. (b) HSV-LacZ group (n = 6–7) had the same aversion threshold as the Sham group. Rats overexpressing CREB (n = 6) show naloxone aversion only when exposed to a high dose (5 mg/kg). (c) HSV-mCREB rats (n = 9) vocalized and jumped in response to lower foot-shock intensities than HSV-CREB rats (n = 9); intermediate jumping threshold was seen in control groups (n = 6–8). (d) The paw licking latency on a hot plate is shorter in HSV-mCREB rats (n = 11) than in HSV-CREB rats (n = 12). Control groups showed intermediate latencies (n = 9–14).
Similar articles
- Antisense-induced reduction in nucleus accumbens cyclic AMP response element binding protein attenuates cocaine reinforcement.
Choi KH, Whisler K, Graham DL, Self DW. Choi KH, et al. Neuroscience. 2006;137(2):373-83. doi: 10.1016/j.neuroscience.2005.10.049. Epub 2005 Dec 15. Neuroscience. 2006. PMID: 16359811 - Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens.
Barrot M, Wallace DL, Bolaños CA, Graham DL, Perrotti LI, Neve RL, Chambliss H, Yin JC, Nestler EJ. Barrot M, et al. Proc Natl Acad Sci U S A. 2005 Jun 7;102(23):8357-62. doi: 10.1073/pnas.0500587102. Epub 2005 May 27. Proc Natl Acad Sci U S A. 2005. PMID: 15923261 Free PMC article. - Molecular mechanisms of drug addiction.
Nestler EJ. Nestler EJ. Neuropharmacology. 2004;47 Suppl 1:24-32. doi: 10.1016/j.neuropharm.2004.06.031. Neuropharmacology. 2004. PMID: 15464123 Review. - The gene transcription factor cyclic AMP-responsive element binding protein: role in positive and negative affective states of alcohol addiction.
Pandey SC. Pandey SC. Pharmacol Ther. 2004 Oct;104(1):47-58. doi: 10.1016/j.pharmthera.2004.08.002. Pharmacol Ther. 2004. PMID: 15500908 Review.
Cited by
- The adenosine A(2A) receptor agonist CGS 21680 alleviates auditory sensorimotor gating deficits and increases in accumbal CREB in rats neonatally treated with quinpirole.
Brown RW, Bhide PG, Gill WD, Peeters LD. Brown RW, et al. Psychopharmacology (Berl). 2020 Dec;237(12):3519-3527. doi: 10.1007/s00213-020-05631-8. Epub 2020 Aug 8. Psychopharmacology (Berl). 2020. PMID: 32772144 Free PMC article. - Transcriptional mechanisms of drug addiction.
Nestler EJ. Nestler EJ. Clin Psychopharmacol Neurosci. 2012 Dec;10(3):136-43. doi: 10.9758/cpn.2012.10.3.136. Epub 2012 Dec 20. Clin Psychopharmacol Neurosci. 2012. PMID: 23430970 Free PMC article. - Changes in nucleus accumbens and neostriatal c-Fos and DARPP-32 immunoreactivity during different stages of food-reinforced instrumental training.
Segovia KN, Correa M, Lennington JB, Conover JC, Salamone JD. Segovia KN, et al. Eur J Neurosci. 2012 Apr;35(8):1354-67. doi: 10.1111/j.1460-9568.2012.08036.x. Epub 2012 Mar 30. Eur J Neurosci. 2012. PMID: 22462413 Free PMC article. - Inhibition of cAMP responsive element binding protein in striatal neurons enhances approach and avoidance responses toward morphine--and morphine withdrawal-related cues.
Sanchis-Segura C, Jancic D, Jimenez-Minchan M, Barco A. Sanchis-Segura C, et al. Front Behav Neurosci. 2009 Sep 8;3:30. doi: 10.3389/neuro.08.030.2009. eCollection 2009. Front Behav Neurosci. 2009. PMID: 19826619 Free PMC article. - Regulation of Brain DNA Methylation Factors and of the Orexinergic System by Cocaine and Food Self-Administration.
Saad L, Sartori M, Pol Bodetto S, Romieu P, Kalsbeek A, Zwiller J, Anglard P. Saad L, et al. Mol Neurobiol. 2019 Aug;56(8):5315-5331. doi: 10.1007/s12035-018-1453-6. Epub 2019 Jan 2. Mol Neurobiol. 2019. PMID: 30603957
References
- Mayr B. & Montminy, M. (2001) Nat. Rev. Mol. Cell Biol. 2, 599-609. - PubMed
- Yin J. C. & Tully, T. (1996) Curr. Opin. Neurobiol. 6, 264-268. - PubMed
- Impey S., Smith, D. M., Obrietan, K., Donahue, R., Wade, C. & Storm, D. R. (1998) Nat. Neurosci. 1, 595-601. - PubMed
- Mayford M. & Kandel, E. R. (1999) Trends Genet. 15, 463-470. - PubMed
- Silva A. J. & Murphy, G. G. (1999) Brain Res. Bull. 50, 441-442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases