Salmonella type III secretion-associated protein InvE controls translocation of effector proteins into host cells - PubMed (original) (raw)
Salmonella type III secretion-associated protein InvE controls translocation of effector proteins into host cells
Tomoko Kubori et al. J Bacteriol. 2002 Sep.
Abstract
Salmonella enterica encodes a type III secretion system (TTSS) within a pathogenicity island located at centisome 63 (SPI-1), which is essential for its pathogenicity. This system mediates the transfer of a battery of bacterial proteins into the host cell with the capacity to modulate cellular functions. The transfer process is dependent on the function of protein translocases SipB, SipC, and SipD. We report here that Salmonella protein InvE, which is also encoded within SPI-1, is essential for the translocation of bacterial proteins into host cells. An S. enterica serovar Typhimurium mutant carrying a loss-of-function mutation in invE shows reduced secretion of SipB, SipC, and SipD while exhibiting increased secretion of other TTSS effector proteins. We also demonstrate that InvE interacts with a protein complex formed by SipB, SipC, and their cognate chaperone, SicA. We propose that InvE controls protein translocation by regulating the function of the Sip protein translocases.
Figures
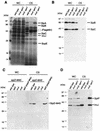
FIG. 1.
S. enterica serovar Typhimurium invE mutant shows altered levels of type III secretion. (A) Coomassie blue staining of total protein of whole-cell lysates (WC; 0.4 ml of culture equivalent) and culture supernatants (CS; 15 ml of culture equivalent) of wild-type, invA, and invE S. enterica serovar Typhimurium strains. (B) Western immunoblot analysis of the levels of SipB and SipC in whole-cell lysates (0.1 ml of culture equivalent) and culture supernatants (0.2 ml of culture equivalent) of the indicated strains. Immunoblots were simultaneously treated with monoclonal antibodies to SipB and SipC. (C) Western immunoblot analysis of the levels of SipD. Whole-cell lysates (1 ml of culture equivalent) and culture supernatants (20 ml of culture equivalent) of the indicated mutant strains encoding a functional M45 epitope-tagged version of SipD in their chromosomes (sipD-M45) or in a plasmid (p_sipD-M45_) were analyzed by Western immunoblotting using an antibody directed to the epitope tag. (D) Western immunoblot analysis of the levels of SptP and InvJ in whole-cell lysates (0.2 ml of culture equivalent) and culture supernatants (2 ml of culture equivalent) of the indicated strains. Immunoblots were simultaneously treated with monoclonal antibodies to SptP and InvJ.
FIG. 2.
Effect of a loss-of-function mutation in invE on protein translocation into Henle-407 cells infected with wild-type or mutant strains of S. enterica serovar Typhimurium. Shown is detection of SipB and SipC (A) and SptP (B) in fractions of Henle-407 cells infected with wild-type and mutant strains of S. enterica serovar Typhimurium. Lanes a, whole-cell lysate of non-cell-associated bacteria; lanes b, bacterium-free infection medium; lanes c, Triton X-100-insoluble fraction containing internalized bacteria; lanes d, Triton X-100-soluble Henle-407 cell lysate containing translocated proteins. SipB, SipC, and SptP were detected by Western immunoblotting with monoclonal antibodies specific for these proteins. The relevant genotypes of the infecting strains are indicated at the top of the gel. The positions of relevant proteins are indicated.
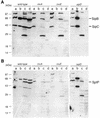
FIG. 3.
InvE exerts its function from within the bacterial cell. (A) Western blot analysis of whole-cell lysates (WC; 0.4 ml of culture equivalent) and cultured supernatants (CS; 16 ml of culture equivalent) of wild-type and mutant S. enterica serovar Typhimurium strains. Blots were treated with a monoclonal antibody specific to InvE. (B) InvE is not susceptible to exogenously applied proteinase K. The indicated bacterial strains were treated with the indicated amounts of proteinase K (ProK) in the presence (+) or absence (−) of 1% SDS, and the levels of InvE after treatment were determined by Western immunoblot analysis with a monoclonal antibody specific to InvE. (C) A GST-InvE chimeric protein can complement the epithelial cell invasion defect of an S. enterica serovar Typhimurium invE mutant strain. The levels of internalization into cultured Henle-407 cells of the indicated strains were determined by the gentamicin protection assay as indicated in Materials and Methods. Values are average percentages of the inoculum that survived the antibiotic treatment and standard deviations from three independent determinations. (D) A GST-InvE chimeric protein can complement the secretion defect of an S. enterica serovar Typhimurium invE mutant strain. Western immunoblot analysis of the levels of SipB and SipC in whole-cell lysates (0.1 ml of culture equivalent) and culture supernatants (0.4 ml of culture equivalent) of the indicated strains. Immunoblots were simultaneously treated with monoclonal antibodies to SipB and SipC. The apparent “rescue” of SipC secretion in the invE mutant carrying a plasmid expressing only GST is due to overexposure of the film and slight lysis induced by this plasmid.
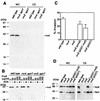
FIG. 4.
Localization of InvE in bacterial cells. (A) Determination of the number of InvE molecules per bacterial cell. The level of InvE in whole-cell lysates (WC; 0.3 ml of culture equivalent) of wild-type S. enterica serovar Typhimurium was determined by Western blot analysis using a monoclonal antibody specific to InvE and compared to the indicated amounts of purified GST-InvE, and the number of molecules was calculated as indicated in Materials and Methods. (B) InvE localizes to both the soluble and pelletable fractions after high-speed centrifugation. Whole-cell extracts from the indicated strains were subjected to high-speed centrifugation. Proteins in the pellet (pellet) and supernatants (sup) after this fractionation along with whole-cell lysates were analyzed by Western immunoblotting with a monoclonal antibody directed to InvE. WC, 0.4 ml of culture equivalent; Sup, 0.8 ml of culture equivalent; pellet, 4 ml of culture equivalent. (C) InvE is localized both to the bacterial membrane and cytosol. Lysates from wild-type and invE S. enterica serovar Typhimurium strains were fractionated on a sucrose gradient as indicated in Materials and Methods, and the presence of InvE in the different fractions was analyzed by Western immunoblotting with a monoclonal antibody directed to InvE. The presence in the different fractions of the membrane (Memb) and cytoplasmic proteins OmpA and 6-phosphogluconate dehydrogenase (6-PD), respectively, was determined by reprobing the blots with antibodies specific to these proteins.
FIG. 5.
invE is epistatic over sipD. Coomassie blue staining of total protein of whole-cell lysates (WC; 0.4 ml of culture equivalent) and culture supernatants (CS; 10 ml of culture equivalent) of the indicated strains. The identities of the polypeptides in culture supernatants are indicated.
FIG. 6.
InvE forms a complex with SicA, SipB, and SipC in S. enterica serovar Typhimurium cell lysates. The presence of SipB and SipC (A), SipD (B), or SicA (C) bound to GST- or GST-InvE-coated beads (pellet, 35 ml of culture equivalent) or free in bacterial lysates before (pre; 0.5 ml of culture equivalent) and after (post; 0.5 ml of culture equivalent) the pull down was detected by immunoblotting with specific monoclonal antibodies to the different proteins (SipB and SipC) or to the M45 epitope tag (SipD and SicA).
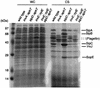
FIG. 7.
InvE interacts with the SicA-SipB and SicA-SipC complexes but not with its individual components. (A to G) Whole-cell lysates from E. coli strains with plasmids carrying sipB, sipC, and sicA either alone or in the indicated combinations were subjected to a GST-InvE pull-down assay as indicated in Materials and Methods. The presence of SipB (A, C, and E), SipC (B, D, and F), or SicA (G) bound to the GST- or GST-InvE-coated beads (pellet) or free in bacterial lysates before (pre) and after (post) the pull down was detected by immunoblotting with specific monoclonal antibodies to the different proteins (SipB and SipC) or to the epitope tag (SicA). The interaction of between InvE and the different proteins was also probed in a yeast two-hybrid assay (H) as indicated in Materials and Methods. The interaction between Legionella pneumophila IcmQ and IcmR was used as a positive control (6). The β-galactosidase activity is expressed in Miller units.
Similar articles
- Molecular characterization of the InvE regulator in the secretion of type III secretion translocases in Salmonella enterica serovar Typhimurium.
Kim JS, Jang JI, Eom JS, Oh CH, Kim HG, Kim BH, Bang IS, Bang SH, Park YK. Kim JS, et al. Microbiology (Reading). 2013 Mar;159(Pt 3):446-461. doi: 10.1099/mic.0.061689-0. Epub 2013 Jan 3. Microbiology (Reading). 2013. PMID: 23288540 - Complex function for SicA, a Salmonella enterica serovar typhimurium type III secretion-associated chaperone.
Tucker SC, Galán JE. Tucker SC, et al. J Bacteriol. 2000 Apr;182(8):2262-8. doi: 10.1128/JB.182.8.2262-2268.2000. J Bacteriol. 2000. PMID: 10735870 Free PMC article. - Role of the Salmonella pathogenicity island 1 (SPI-1) protein InvB in type III secretion of SopE and SopE2, two Salmonella effector proteins encoded outside of SPI-1.
Ehrbar K, Friebel A, Miller SI, Hardt WD. Ehrbar K, et al. J Bacteriol. 2003 Dec;185(23):6950-67. doi: 10.1128/JB.185.23.6950-6967.2003. J Bacteriol. 2003. PMID: 14617659 Free PMC article. - Characterization of effector proteins translocated via the SPI1 type III secretion system of Salmonella typhimurium.
Ehrbar K, Mirold S, Friebel A, Stender S, Hardt WD. Ehrbar K, et al. Int J Med Microbiol. 2002 Feb;291(6-7):479-85. doi: 10.1078/1438-4221-00156. Int J Med Microbiol. 2002. PMID: 11890547 Review. - The role of host cell death in Salmonella infections.
Guiney DG. Guiney DG. Curr Top Microbiol Immunol. 2005;289:131-50. doi: 10.1007/3-540-27320-4_6. Curr Top Microbiol Immunol. 2005. PMID: 15791954 Review.
Cited by
- Role of Salmonella Pathogenicity Island 1 protein IacP in Salmonella enterica serovar typhimurium pathogenesis.
Kim JS, Eom JS, Jang JI, Kim HG, Seo DW, Bang IS, Bang SH, Lee IS, Park YK. Kim JS, et al. Infect Immun. 2011 Apr;79(4):1440-50. doi: 10.1128/IAI.01231-10. Epub 2011 Jan 24. Infect Immun. 2011. PMID: 21263021 Free PMC article. - YopN Is Required for Efficient Effector Translocation and Virulence in Yersinia pseudotuberculosis.
Bamyaci S, Ekestubbe S, Nordfelth R, Erttmann SF, Edgren T, Forsberg Å. Bamyaci S, et al. Infect Immun. 2018 Jul 23;86(8):e00957-17. doi: 10.1128/IAI.00957-17. Print 2018 Aug. Infect Immun. 2018. PMID: 29760214 Free PMC article. - Steps for Shigella Gatekeeper Protein MxiC Function in Hierarchical Type III Secretion Regulation.
Roehrich AD, Bordignon E, Mode S, Shen DK, Liu X, Pain M, Murillo I, Martinez-Argudo I, Sessions RB, Blocker AJ. Roehrich AD, et al. J Biol Chem. 2017 Feb 3;292(5):1705-1723. doi: 10.1074/jbc.M116.746826. Epub 2016 Dec 14. J Biol Chem. 2017. PMID: 27974466 Free PMC article. - CesL Regulates Type III Secretion Substrate Specificity of the Enteropathogenic E. coli Injectisome.
Díaz-Guerrero M, Gaytán MO, Soto E, Espinosa N, García-Gómez E, Marcos-Vilchis A, Andrade A, González-Pedrajo B. Díaz-Guerrero M, et al. Microorganisms. 2021 May 13;9(5):1047. doi: 10.3390/microorganisms9051047. Microorganisms. 2021. PMID: 34067942 Free PMC article.
References
- Allaoui, A., P. J. Sansonetti, and C. Parsot. 1993. MxiD, an outer membrane protein necessary for the secretion of the Shigella flexneri Ipa invasins. Mol. Microbiol. 7:59-68. - PubMed
- Anderson, D. M., and O. Schneewind. 1997. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 278:1140-1143. - PubMed
- Anderson, D. M., and O. Schneewind. 1999. Yersinia enterocolitica type III secretion: an mRNA signal that couples translation and secretion of YopQ. Mol. Microbiol. 31:1139-1148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous