Myelin basic protein-primed T cells induce nitric oxide synthase in microglial cells. Implications for multiple sclerosis - PubMed (original) (raw)
Myelin basic protein-primed T cells induce nitric oxide synthase in microglial cells. Implications for multiple sclerosis
Subhajit Dasgupta et al. J Biol Chem. 2002.
Abstract
The presence of autoreactive T cells recognizing self myelin antigens is necessary for the development of central nervous system autoimmune diseases such as multiple sclerosis (MS). The present study was undertaken to investigate the role of myelin basic protein (MBP)-primed T cells in the expression of inducible nitric oxide synthase (iNOS) in microglial cells. MBP-primed T cells alone markedly induced the production of NO and the expression of iNOS protein and mRNA in mouse BV-2 microglial cells. Similarly, MBP-primed T cells also induced the production of NO in mouse primary microglia. This induction of NO production was primarily dependent on the contact between MBP-primed T cells and microglia. The expression of very late antigen-4 (VLA-4) on the surface of MBP-primed T cells and inhibition of MBP-primed T cell-induced microglial NO production by functional blocking of antibodies to the alpha(4) chain of VLA-4 (CD49d) suggest that VLA-4 integrin on MBP-primed T cells plays an important role in contact-mediated induction of iNOS. Since IFN-beta has been used to treat MS patients, we examined the effect of IFN-beta on MBP-primed T cell-induced the production of NO. Surprisingly, IFN-beta alone induced the production of NO in microglial cells. However, the pretreatment of MBP-primed T cells with IFN-beta inhibited the expression of VLA-4 integrin on the surface of MBP-primed T cells and thereby inhibited the ability of those T cells to induce the production of NO in microglial cells. This study illustrates a novel role of neuroantigen-primed T cells in inducing contact-mediated expression of iNOS in microglial cells that may participate in the pathogenesis of MS.
Figures
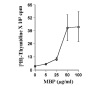
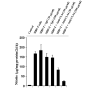
Fig. 1. Proliferation of T lymphocytes with MBP
T cells suspended in RPMI containing 10% FBS were treated with different concentrations of MBP. After 4 days of incubation, proliferation was assayed using [3H]thymidine as described under “Materials and Methods.” Data are mean ± S.D. of three different experiments.
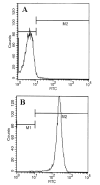
Fig. 2. FACS analysis of T lymphocytes with FITC-labeled anti-CD3
Purified T cells were treated with 0.5 ml of appropriately diluted FITC-labeled anti-CD3 for 30 min followed by FACS analysis in a FACScan as described under “Materials and Methods.” A, FITC-unconjugated; B, FITC-conjugated.
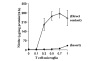
Fig. 3. MBP-primed T lymphocytes induce the production of NO in mouse BV-2 microglial cells
BV-2 cells received different concentrations of MBP-primed T cells in direct contact or within insert under serum-free conditions. After 24 h, supernatants were used for nitrite assay as described under “Materials and Methods.” Data are mean ± S.D. of three different experiments.
Fig. 4. MBP-primed T lymphocytes induce the expression of iNOS in mouse BV-2 microglial cells
BV-2 cells were stimulated with different concentrations of MBP-primed T cells under serum-free conditions. In A, cell homogenates were electrophoresed, transferred on nitrocellulose membrane, and immunoblotted with antibodies against mouse macrophage iNOS as described under “Materials and Methods.” In B, after 6 h of incubation, Northern blot analysis for iNOS mRNA was carried out as described under “Materials and Methods.” GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
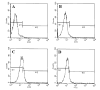
Fig. 5. Expression of VLA4 on the surface of MBP-primed T cells
Normal or MBP-primed T cells were treated with 0.5 ml of appropriately diluted FITC-labeled anti-VLA _α_4 for 30 min followed by FACS analysis. A, FITC-unconjugated normal T cells; B, FITC-conjugated normal T cells; C, FITC-conjugated MBP-primed T cells. In D, similarly MBP-primed T cells that were treated with 50 units/ml IFN-β during priming with 50 _μ_g/ml MBP for 4 days were analyzed by FACS.
Fig. 6. Functional blocking antibodies against the _α_4 chain of VLA4 inhibit the ability of MBP-primed T cells to induce the production of NO in BV-2 microglial cells
MBP-primed T cells were mixed with either different concentrations of antibodies against the _α_4 chain of VLA4 or control IgG and rocked gently for 1 h at room temperature. Cells were centrifuged, washed twice, and added to BV-2 microglial cells at a ratio of 0.7:1 T cell:microglia. After 24 h of stimulation, supernatants were used for nitrite assay. Data are mean ± S.D. of three different experiments.
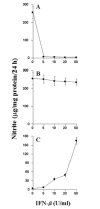
Fig. 7. Effect of IFN-β on MBP-primed T cell-induced production of NO in BV-2 microglial cells
BV-2 cells were stimulated under serum-free conditions with MBP-primed T cells, which were pretreated with different concentrations of IFN-β (A), MBP-primed T cells followed by the treatment of different concentrations of IFN-β (B), and different concentrations of IFN-β (C). After 24 h of stimulation, supernatants were used for nitrite assay. Data are mean ± S.D. of three different experiments.
Similar articles
- Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells.
Dasgupta S, Jana M, Liu X, Pahan K. Dasgupta S, et al. J Biol Chem. 2003 Jun 20;278(25):22424-31. doi: 10.1074/jbc.M301789200. Epub 2003 Apr 10. J Biol Chem. 2003. PMID: 12690109 Free PMC article. - Myelin basic protein-primed T cells of female but not male mice induce nitric-oxide synthase and proinflammatory cytokines in microglia: implications for gender bias in multiple sclerosis.
Dasgupta S, Jana M, Liu X, Pahan K. Dasgupta S, et al. J Biol Chem. 2005 Sep 23;280(38):32609-17. doi: 10.1074/jbc.M500299200. Epub 2005 Jul 26. J Biol Chem. 2005. PMID: 16046404 Free PMC article. - Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells.
Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Jana M, et al. J Biol Chem. 2001 Nov 30;276(48):44527-33. doi: 10.1074/jbc.M106771200. Epub 2001 Sep 10. J Biol Chem. 2001. PMID: 11551948 Free PMC article. - Gender-specific expression of beta1 integrin of VLA-4 in myelin basic protein-primed T cells: implications for gender bias in multiple sclerosis.
Brahmachari S, Pahan K. Brahmachari S, et al. J Immunol. 2010 Jun 1;184(11):6103-13. doi: 10.4049/jimmunol.0804356. Epub 2010 May 5. J Immunol. 2010. PMID: 20483780 Free PMC article. - Multiple Sclerosis: New Insights into Molecular Pathogenesis and Novel Platforms for Disease Treatment.
Dejbakht M, Akhzari M, Jalili S, Faraji F, Barazesh M. Dejbakht M, et al. Curr Drug Res Rev. 2024;16(2):175-197. doi: 10.2174/2589977516666230915103730. Curr Drug Res Rev. 2024. PMID: 37724675 Review.
Cited by
- Suppression of Experimental Autoimmune Encephalomyelitis in Mice by β-Hydroxy β-Methylbutyrate, a Body-Building Supplement in Humans.
Sheinin M, Mondal S, Roy A, Gorai S, Rangasamy SB, Poddar J, Pahan K. Sheinin M, et al. J Immunol. 2023 Jul 15;211(2):187-198. doi: 10.4049/jimmunol.2200267. J Immunol. 2023. PMID: 37314416 Free PMC article. - CD4+ T cell expression of the IL-10 receptor is necessary for facial motoneuron survival after axotomy.
Runge EM, Iyer AK, Setter DO, Kennedy FM, Sanders VM, Jones KJ. Runge EM, et al. J Neuroinflammation. 2020 Apr 17;17(1):121. doi: 10.1186/s12974-020-01772-x. J Neuroinflammation. 2020. PMID: 32303238 Free PMC article. - Microglia in Multiple Sclerosis: Friend or Foe?
Guerrero BL, Sicotte NL. Guerrero BL, et al. Front Immunol. 2020 Mar 20;11:374. doi: 10.3389/fimmu.2020.00374. eCollection 2020. Front Immunol. 2020. PMID: 32265902 Free PMC article. Review. - The Neuroimmune and Neurotoxic Fingerprint of Major Neurocognitive Psychosis or Deficit Schizophrenia: a Supervised Machine Learning Study.
Al-Hakeim HK, Almulla AF, Maes M. Al-Hakeim HK, et al. Neurotox Res. 2020 Mar;37(3):753-771. doi: 10.1007/s12640-019-00112-z. Epub 2020 Jan 8. Neurotox Res. 2020. PMID: 31916129 - Low-Dose Maraviroc, an Antiretroviral Drug, Attenuates the Infiltration of T Cells into the Central Nervous System and Protects the Nigrostriatum in Hemiparkinsonian Monkeys.
Mondal S, Rangasamy SB, Roy A, Dasarathy S, Kordower JH, Pahan K. Mondal S, et al. J Immunol. 2019 Jun 15;202(12):3412-3422. doi: 10.4049/jimmunol.1800587. J Immunol. 2019. PMID: 31043478 Free PMC article.
References
- Nathan C. FASEB J. 1992;6:3051–3064. - PubMed
- Jaffrey SR, Snyder SH. Annu Rev Cell Dev Biol. 1995;11:417–440. - PubMed
- Mitrovic B, Ignarro LJ, Montestruque S, Smoll A, Merril JE. Neuroscience. 1994;61:575–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous