SNAREs in native plasma membranes are active and readily form core complexes with endogenous and exogenous SNAREs - PubMed (original) (raw)
SNAREs in native plasma membranes are active and readily form core complexes with endogenous and exogenous SNAREs
Thorsten Lang et al. J Cell Biol. 2002.
Abstract
During neuronal exocytosis, the vesicle-bound soluble NSF attachment protein (SNAP) receptor (SNARE) synaptobrevin 2 forms complexes with the plasma membrane-bound SNAREs syntaxin 1A and SNAP25 to initiate the fusion reaction. However, it is not known whether in the native membrane SNAREs are constitutively active or whether they are unable to enter SNARE complexes unless activated before membrane fusion. Here we used binding of labeled recombinant SNAREs to inside-out carrier supported plasma membrane sheets of PC12 cells to probe for the activity of endogenous SNAREs. Binding was specific, saturable, and depended on the presence of membrane-resident SNARE partners. Our data show that virtually all of the endogenous syntaxin 1 and SNAP-25 are highly reactive and readily form SNARE complexes with exogenously added SNAREs. Furthermore, complexes between endogenous SNAREs were not detectable when the membranes are freshly prepared, but they slowly form upon prolonged incubation in vitro. We conclude that the activity of membrane-resident SNAREs is not downregulated by control proteins but is constitutively active even if not engaged in fusion events.
Figures
Figure 1.
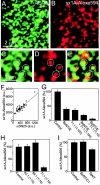
Syntaxin 1A binds to endogenous SNAP-25 on inside-out sheets of plasma membrane derived from PC12 cells. (A and B) Membrane sheets were reacted for 50 min at 37°C with syntaxin 1A(1–262)–Alexa594 (red channel) and then washed, fixed, and immunostained for SNAP-25 (green channel). (C and D) Magnified views from A and B. (E) Overlap from C and D. Circles indicate identical pixel locations. (F) On membrane sheets such as shown in A and B the staining intensity of random images in the green channel was measured and plotted against the staining intensity in the red channel, showing a linear correlation. (G) Effect of SNAP-25 cleavage by 2 μM of BoNT/E and/or Cl 71.1 (antibody specific for SNAP-25) on syntaxin binding, measured as above. Dilution of the antibody as indicated. (H) Effect of antibodies against rab3 (Cl 42.1), synaptobrevin 2 (Cl 69.1), and SNAP-25 (Cl 71.1) on syntaxin binding. (I) Binding of syntaxin in the presence of 2 μM BoNT/C1 (cleaving syntaxin) and 2 μM TeNT (cleaving synaptobrevin).
Figure 4.
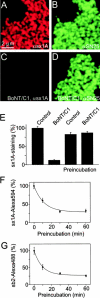
Endogenous syntaxin 1 is quantitatively cleaved by BoNT/C1 on freshly isolated inside-out membrane sheets but not after prolonged incubation suggesting formation of SNARE core complexes over time. (A–D) Membrane sheets treated for 45 min in the presence (C and D) or absence (A and B) of 5 μM BoNT/C1 were immunostained for syntaxin 1A/B (A and C) and SNAP-25 (B and D). (E) Quantification of syntaxin-immunostaining intensity on membrane sheets as shown in A and C and membrane sheets that were incubated for 1 h before BoNT/C1 treatment. Binding of syntaxin 1A–Alexa594 (F) and synaptobrevin 2–Alexa488 (G) to membrane sheets that were preincubated at 37°C in buffer for 0, 10, 30, or 60 min before addition of the labeled proteins.
Figure 2.
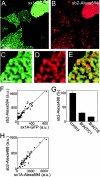
Synaptobrevin 2 binds to endogenous syntaxin clusters on inside-out membrane sheets but also requires endogenous SNAP-25 for binding. (A and B) Membrane sheets were produced from cells expressing syntaxin 1A-GFP (green channel), and then reacted with synaptobrevin 2–Alexa594 (red channel). (C and D) Magnified views from A and B. (E) Overlap from C and D. (F) Plotting green against red fluorescence intensity from membrane sheets as shown in A and B. (G) Synaptobrevin 2 binding depends both on intact syntaxin 1 and SNAP-25, as it is inhibited by membrane treatment with either 2 μM BoNT/C1 or BoNT/E. (H) Membrane sheets were reacted with syntaxin 1A–Alexa594 for 60 min, washed two times at room temperature in BSA-KGlu buffer, and then reacted for 50 min with synaptobrevin 2–Alexa488. In this experiment, the measured green fluorescence intensity is an underestimate due to FRET occurring from the green to the red label. Note that in the experiments shown in G and H, nontransfected cells were used.
Figure 3.
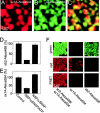
Syntaxin 1A–Alexa594 and synaptobrevin 2–Alexa488 form SNARE complexes on inside-out membrane sheets. (A–C) Membrane sheets reacted simultaneously with syntaxin 1A–Alexa594 (red channel) and synaptobrevin 2–Alexa488 (green channel). (D and E) NSF removes bound syntaxin 1 and synaptobrevin 2 from inside-out membranes. Membrane sheets reacted for 50 min with either synaptobrevin 2–Alexa488 (D) or syntaxin 1A–Alexa594 (E) were incubated with 0.5 μg/μl αSNAP and 0.1 μg/μl NSF during the last 10 min of the binding reaction, either in the presence of ATP or in the absence of ATP (including 1 mM EDTA). (F) Membrane sheets were reacted with syntaxin 1A–Alexa594 and synaptobrevin 2–Alexa488 either together (left) or alone (middle, right) and viewed in the green, red or the FRET channel. When both labeled proteins are bound a strong signal is detected in the FRET channel.
Figure 5.
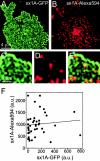
Binding of exogenous syntaxin 1A is not affected by syntaxin overexpression. Syntaxin 1A–Alexa594 binding was measured on membrane sheets from cells expressing syntaxin 1A–GFP. (A) Syntaxin 1A–GFP (green channel). (B) Syntaxin 1A–Alexa594 (red channel). (C and D) Magnified views from A and B. (E) Overlap from C and D. (F) Plotting green against red fluorescence intensity in random images obtained from membrane sheets as shown in A and B.
Figure 6.
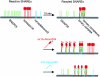
Working model: in the native plasma membrane the SNAREs are reactive. When isolated plasma membrane sheets age, the reactive endogenous SNAREs form binary and ternary complexes. Reactive endogenous SNAREs also form SNARE complexes with added recombinant SNAREs.
Similar articles
- A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function.
Antonin W, Holroyd C, Fasshauer D, Pabst S, Von Mollard GF, Jahn R. Antonin W, et al. EMBO J. 2000 Dec 1;19(23):6453-64. doi: 10.1093/emboj/19.23.6453. EMBO J. 2000. PMID: 11101518 Free PMC article. - Regulation of neuronal SNARE assembly by the membrane.
Kweon DH, Kim CS, Shin YK. Kweon DH, et al. Nat Struct Biol. 2003 Jun;10(6):440-7. doi: 10.1038/nsb928. Nat Struct Biol. 2003. PMID: 12740606 - Fusion of cells by flipped SNAREs.
Hu C, Ahmed M, Melia TJ, Söllner TH, Mayer T, Rothman JE. Hu C, et al. Science. 2003 Jun 13;300(5626):1745-9. doi: 10.1126/science.1084909. Science. 2003. PMID: 12805548 - Fusion of membranes during the acrosome reaction: a tale of two SNAREs.
Kierszenbaum AL. Kierszenbaum AL. Mol Reprod Dev. 2000 Dec;57(4):309-10. doi: 10.1002/1098-2795(200012)57:4<309::AID-MRD1>3.0.CO;2-W. Mol Reprod Dev. 2000. PMID: 11066058 Review. - Regulation of growth cone extension by SNARE proteins.
Kimura K, Mizoguchi A, Ide C. Kimura K, et al. J Histochem Cytochem. 2003 Apr;51(4):429-33. doi: 10.1177/002215540305100404. J Histochem Cytochem. 2003. PMID: 12642621 Review.
Cited by
- Munc18-1 protein molecules move between membrane molecular depots distinct from vesicle docking sites.
Smyth AM, Yang L, Martin KJ, Hamilton C, Lu W, Cousin MA, Rickman C, Duncan RR. Smyth AM, et al. J Biol Chem. 2013 Feb 15;288(7):5102-13. doi: 10.1074/jbc.M112.407585. Epub 2012 Dec 6. J Biol Chem. 2013. PMID: 23223447 Free PMC article. - PRIP (phospholipase C-related but catalytically inactive protein) inhibits exocytosis by direct interactions with syntaxin 1 and SNAP-25 through its C2 domain.
Zhang Z, Takeuchi H, Gao J, Wang D, James DJ, Martin TFJ, Hirata M. Zhang Z, et al. J Biol Chem. 2013 Mar 15;288(11):7769-7780. doi: 10.1074/jbc.M112.419317. Epub 2013 Jan 22. J Biol Chem. 2013. PMID: 23341457 Free PMC article. - Syntaxins 3 and 4 are concentrated in separate clusters on the plasma membrane before the establishment of cell polarity.
Low SH, Vasanji A, Nanduri J, He M, Sharma N, Koo M, Drazba J, Weimbs T. Low SH, et al. Mol Biol Cell. 2006 Feb;17(2):977-89. doi: 10.1091/mbc.e05-05-0462. Epub 2005 Dec 7. Mol Biol Cell. 2006. PMID: 16339081 Free PMC article. - The specificity of SNARE pairing in biological membranes is mediated by both proof-reading and spatial segregation.
Bethani I, Lang T, Geumann U, Sieber JJ, Jahn R, Rizzoli SO. Bethani I, et al. EMBO J. 2007 Sep 5;26(17):3981-92. doi: 10.1038/sj.emboj.7601820. Epub 2007 Aug 23. EMBO J. 2007. PMID: 17717530 Free PMC article. - t-SNARE protein conformations patterned by the lipid microenvironment.
Rickman C, Medine CN, Dun AR, Moulton DJ, Mandula O, Halemani ND, Rizzoli SO, Chamberlain LH, Duncan RR. Rickman C, et al. J Biol Chem. 2010 Apr 30;285(18):13535-41. doi: 10.1074/jbc.M109.091058. Epub 2010 Jan 21. J Biol Chem. 2010. PMID: 20093362 Free PMC article.
References
- Aguado, F., G. Majo, B. Ruiz-Montasell, J.M. Canals, A. Casanova, J. Marsal, and J. Blasi. 1996. Expression of synaptosomal-associated protein SNAP-25 in endocrine anterior pituitary cells. Eur. J. Cell Biol. 69:351–359. - PubMed
- Antonin, W., D. Fasshauer, S. Becker, R. Jahn, and T.R. Schneider. 2002. Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat. Struct. Biol. 9:107–111. - PubMed
- Avery, J., R. Jahn, and J.M. Edwardson. 1999. Reconstitution of regulated exocytosis in cell-free systems: a critical appraisal. Annu. Rev. Physiol. 61:777–807. - PubMed
- Banerjee, A., V.A. Barry, B.R. DasGupta, and T.F. Martin. 1996. N-Ethylmaleimide-sensitive factor acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J. Biol. Chem. 271:20223–20226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases