Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism - PubMed (original) (raw)
Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism
David B Carr et al. J Neurosci. 2002.
Abstract
The serotonin (5-HT) innervation of the prefrontal cortex (PFC) exerts a powerful modulatory influence on neuronal activity in this cortical region, although the mechanisms through which 5-HT modulates cellular activity are unclear. Voltage-dependent Na+ channels are one potential target of 5-HT receptor signaling that have wide-ranging effects on activity. Molecular and electrophysiological studies were used to test this potential linkage. Single cell RT-PCR profiling revealed that the vast majority of pyramidal neurons expressed detectable levels of 5-HT2a and/or 5-HT2c receptor mRNA with half of the cells expressing both mRNAs. Whole-cell voltage-clamp recordings of dissociated pyramidal neurons showed that 5-HT2a/c receptor activation reduced rapidly inactivating Na+ currents by reducing maximal current amplitude and shifting fast inactivation voltage dependence. These effects were mediated by G(q) activation of phospholipase C, leading to activation of protein kinase C (PKC). 5-HT2a/c receptor stimulation also reduced the amplitude of persistent Na+ current without altering its activation voltage dependence. This modulation was also mediated by PKC. Although 5-HT(2a,c) receptor activation did not affect somatic action potentials of layer V pyramidal neurons in PFC slices, it did reduce the amplitude of action potentials backpropagating into the apical dendrite. These findings show that 5-HT2a,c receptor activation reduces dendritic excitability and may negatively modulate activity-dependent dendritic synaptic plasticity.
Figures
Fig. 1.
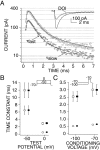
5-HT2a/c receptor stimulation reduces rapidly inactivating Na+ current in PFC pyramidal cells. A, Plot of peak Na+ current evoked by a step from a holding potential of −70 to −35 mV as a function of time. Application of 5-HT (1 μ
m
) reversibly suppressed peak Na+ current. B, Dose–response curve for 5-HT-mediated reduction in current. The curve represents the least squares fit of a Langmuir isotherm to the data with a EC50 of 132 n
m
. C, Single-cell RT-PCR revealed that the majority of PFC neurons express 5-HT2a and/or 5-HT2c receptor mRNA. In a representative gel, a single PFC pyramidal cell expresses mRNA for Ca2+–calmodulin kinase II (CaMKII) as well as amplicons for both 5-HT2a and 5-HT2c. Frequency distribution of mRNA for CaMKII, 5HT2a, and 5HT2c receptors in 46 PFC pyramidal cells.D, Box plot summary of the effect of 5-HT agonists and antagonists on peak Na+ current. The effect of 5-HT (1 μ
m
, n = 10) was significantly reduced when coapplied with the 5HT2a/c antagonist spiperone (1 μ
m
, n = 8). The effect of 5-HT was mimicked by the 5-HT2a/c agonist DOI (10 μ
m
, n = 19).
Fig. 2.
5-HT2a/c receptor stimulation shifts the voltage dependence of steady-state inactivation to more negative potentials. A, In a representative cell, TTX-subtracted Na+ currents evoked by a step to −10 mV from a range of conditioning voltages (inset).B, Currents from the same cell in the presence of 1 μ
m
5-HT. C, Steady-state inactivation plot showing the peak amplitude of the Na+ currents shown in A (open circles) and B(closed circles). 5-HT (1 μ
m
) reduced peak Na+ current and shifted the voltage dependence of steady-state inactivation to more negative potentials.D, The negative shift in _V_1/2is more pronounced when plotting the normalized peak current (I/I_max) of the same data shown in_C. Inset, Box plot summary of the shift in _V_1/2 produced by 5-HT (1 μ
m
, n = 6) and DOI (10 μ
m
, n = 14).
Fig. 3.
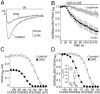
5-HT2a/c receptor stimulation does not alter the inactivation time constants of rapidly inactivating Na+ currents. A, Current traces evoked by a step to −10 mV from a holding potential of −70 mV in the presence (filled circles) or absence (open circles) of 10 μ
m
DOI. Current amplitude is plotted on an inverted semi-log scale to illustrate the biexponential character of the current inactivation. The same traces are also shown in the inset in a more standard form for comparison purposes. The control curve (top line) represents the best fit of a modified Hodgkin–Huxley model (see Materials and Methods). The DOI curve (bottom line) uses the values of activation and inactivation time constants obtained from the control curve, changing only _g_max and_I_NaP. The SE of the resulting curve does not differ significantly from the control curve. B,C, Fast (circles) and slow (squares) inactivation time constants obtained by applying similar curve fits to currents derived from activation (B) and inactivation (C) protocols in the presence (filled symbols) and absence (open symbols) of 10 μ
m
DOI. DOI did not produce a significant change in either the fast or slow inactivation time constants of Na+ currents obtained by either voltage protocol (n = 5). Two representative test voltages (B) or conditioning voltages (C) are presented in each graph.
Fig. 4.
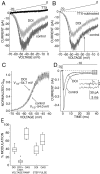
5-HT2a/c receptor modulation of peak Na+ current depends on activation of Gαq and PLC. A, In neurons dialyzed with a peptide (100 μ
m
) that blocks binding of Gαq, DOI (10 μ
m
) failed to significantly reduce peak Na+ current.B, Box plot summary of the reduction in peak Na+ current by DOI in cells dialyzed with the Gαq peptide (n = 8) or a nonsense peptide of equal size (n = 8). C, Blockade of Gαq also prevents the negative shift in the voltage dependence of steady-state inactivation produced by DOI.Inset, Box plot summary of the shift in_V_1/2 by DOI in cells dialyzed with the Gαq peptide (n = 7) or a nonsense peptide (n = 8). D, Dialysis with the PLC inhibitor U-73122 (20 μ
m
) blocks the reduction in peak Na+ current by DOI. U-73122 (0.2 μ
m
) was also included in the extracellular bathing solution. E, Box plot summary of the reduction in peak Na+ current by DOI in cells dialyzed with U-73122 (n = 8) or the weak PLC inhibitor U-73343 (n = 7). F, Dialysis with U-73122 also prevents the negative shift in the voltage dependence of steady-state inactivation induced by 5-HT2a/c receptor activation. Inset, Box plot summary of the shift in_V_1/2 by DOI in cells dialyzed with U-73122 (n = 5) or the weak PLC inhibitor U-73343 (n = 6).
Fig. 5.
5-HT2a/c receptor modulation of peak Na+ current depends on activation of PKC and elevation in intracellular calcium. A, D, Dialysis with the PKC inhibitor calphostin C (1 μ
m
) or with the calcium chelator BAPTA (20 m
m
) blocks the reduction in peak Na+ current by DOI (10 μ
m
). B, E, Box plot summaries of the reduction in peak Na+ current by DOI in control cells (n = 19) and in cells dialyzed with calphostin (n = 6) in B or with BAPTA (n = 9) in E. C, F, Dialysis with either calphostin or BAPTA also prevents the negative shift in the voltage dependence of steady-state inactivation induced by 5-HT2a/c receptor activation.Insets, Box plot summaries of the shift in_V_1/2 by DOI in control cells (n = 14) and in cells dialyzed with calphostin (n = 6) in C or with BAPTA (n = 5) in F.
Fig. 6.
PKC stimulation reduces rapidly inactivating Na+ current and produces a negative shift in the voltage dependence of steady-state inactivation. A, A representative cell showing the reduction in Na+current after a 2 min exposure to the PKC activator OAG (2 μ
m
). B, Plot of average (mean ± SEM) normalized (_I/I_max) peak Na+ current evoked by a step from a holding potential of −70 to −35 mV as a function of time. OAG markedly reduced peak Na+ current during a 2 min exposure (filled circles, n = 6). This reduction was significantly reduced in cells dialyzed with the PKC inhibitor calphostin C (1 μ
m
, open circles, n = 6). C, A representative inactivation plot showing peak Na+current evoked by a step to −10 mV from a range of conditioning voltages. After a 2 min OAG exposure, the peak Na+current evoked from all conditioning potentials was significantly reduced. D, A plot of normalized (_I/I_max) data from _C_more clearly shows a significant negative shift in the voltage dependence of inactivation by OAG. Inset, Box plot summary of the shift in _V_1/2 produced by OAG (n = 6).
Fig. 7.
5-HT2a/c receptor stimulation reduces persistent Na+ current via a PKC-dependent mechanism. A, DOI (10 μ
m
) reduced persistent Na+ current evoked in 115 m
m
external Na+ by a 2 sec voltage ramp from a holding potential of −70 to 0 mV. B, TTX subtracted currents from the cell in A. The persistent current amplitude was measured at −25 mV (arrow) to avoid contamination by window current. C, DOI did not alter the activation kinetics of the persistent current. D, DOI also reduced the amplitude of persistent Na+ current measured at the end of a 40 msec step pulse in 10 m
m
external Na+. The persistent component in the boxed area is magnified to more clearly show the reduced amplitude of DOI (*) compared with control (**) traces. E, Box plot summary of the modulation of persistent Na+ current by DOI measured by voltage ramps (n = 10) or by a step pulse (n = 10). Dialysis with the PKC inhibitor calphostin C (1 μ
m
) significantly attenuated the DOI effect on persistent current evoked by voltage ramps (n = 7). The PKC activator OAG (2 μ
m
) also markedly reduced persistent Na+ current measured by either step pulses (n = 5) or voltage ramps (n = 6).
Fig. 8.
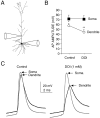
5-HT2a/c receptor stimulation reduces the amplitude of backpropagating action potentials in the apical dendrites of PFC pyramidal neurons. A, Reconstruction of a PFC layer V pyramidal neuron from which simultaneous somatic and dendritic (110 μm) recordings were obtained. B, The amplitude of dendritic action potentials was significantly smaller than somatic action potentials (*p < 0.03;n = 6). DOI (1 μ
m
) reduced the amplitude of backpropagating dendritic action potentials without affecting somatic spike amplitude (**p < 0.01;n = 6). C, Example of simultaneous recordings from the cell shown in B. Note the reduction of dendritic action potential amplitude in DOI, which also produced a slowing of the action potential rise time in the dendrite but not in the soma.
Similar articles
- Serotonin receptors modulate GABA(A) receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons.
Feng J, Cai X, Zhao J, Yan Z. Feng J, et al. J Neurosci. 2001 Sep 1;21(17):6502-11. doi: 10.1523/JNEUROSCI.21-17-06502.2001. J Neurosci. 2001. PMID: 11517239 Free PMC article. - Stimulation of 5-HT(2) receptors in prefrontal pyramidal neurons inhibits Ca(v)1.2 L type Ca(2+) currents via a PLCbeta/IP3/calcineurin signaling cascade.
Day M, Olson PA, Platzer J, Striessnig J, Surmeier DJ. Day M, et al. J Neurophysiol. 2002 May;87(5):2490-504. doi: 10.1152/jn.00843.2001. J Neurophysiol. 2002. PMID: 11976386 - Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors.
Béïque JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Béïque JC, et al. J Neurosci. 2004 May 19;24(20):4807-17. doi: 10.1523/JNEUROSCI.5113-03.2004. J Neurosci. 2004. PMID: 15152041 Free PMC article. - Serotonergic regulation of neuronal excitability in the prefrontal cortex.
Andrade R. Andrade R. Neuropharmacology. 2011 Sep;61(3):382-6. doi: 10.1016/j.neuropharm.2011.01.015. Epub 2011 Jan 18. Neuropharmacology. 2011. PMID: 21251917 Free PMC article. Review. - Molecular and cellular mechanisms for the polarized sorting of serotonin receptors: relevance for genesis and treatment of psychosis.
Roth BL, Xia Z. Roth BL, et al. Crit Rev Neurobiol. 2004;16(4):229-36. doi: 10.1615/critrevneurobiol.v16.i4.10. Crit Rev Neurobiol. 2004. PMID: 15862107 Review.
Cited by
- Distinct 5-HT receptor subtypes regulate claustrum excitability by serotonin and the psychedelic, DOI.
Anderson TL, Keady JV, Songrady J, Tavakoli NS, Asadipooya A, Neeley RE, Turner JR, Ortinski PI. Anderson TL, et al. Prog Neurobiol. 2024 Sep;240:102660. doi: 10.1016/j.pneurobio.2024.102660. Epub 2024 Aug 31. Prog Neurobiol. 2024. PMID: 39218140 - Cellular rules underlying psychedelic control of prefrontal pyramidal neurons.
Ekins TG, Brooks I, Kailasa S, Rybicki-Kler C, Jedrasiak-Cape I, Donoho E, Mashour GA, Rech J, Ahmed OJ. Ekins TG, et al. bioRxiv [Preprint]. 2023 Oct 23:2023.10.20.563334. doi: 10.1101/2023.10.20.563334. bioRxiv. 2023. PMID: 37961554 Free PMC article. Preprint. - Cinacalcet inhibition of neuronal action potentials preferentially targets the fast inactivated state of voltage-gated sodium channels.
Lindner JS, Rajayer SR, Martiszus BJ, Smith SM. Lindner JS, et al. Front Physiol. 2022 Dec 19;13:1066467. doi: 10.3389/fphys.2022.1066467. eCollection 2022. Front Physiol. 2022. PMID: 36601343 Free PMC article. - Mechanistic modeling as an explanatory tool for clinical treatment of chronic catatonia.
Roberts PD, Conour J. Roberts PD, et al. Front Pharmacol. 2022 Nov 9;13:1025417. doi: 10.3389/fphar.2022.1025417. eCollection 2022. Front Pharmacol. 2022. PMID: 36438845 Free PMC article. - Impact of schizophrenia GWAS loci converge onto distinct pathways in cortical interneurons vs glutamatergic neurons during development.
Liu D, Zinski A, Mishra A, Noh H, Park GH, Qin Y, Olorife O, Park JM, Abani CP, Park JS, Fung J, Sawaqed F, Coyle JT, Stahl E, Bendl J, Fullard JF, Roussos P, Zhang X, Stanton PK, Yin C, Huang W, Kim HY, Won H, Cho JH, Chung S. Liu D, et al. Mol Psychiatry. 2022 Oct;27(10):4218-4233. doi: 10.1038/s41380-022-01654-z. Epub 2022 Jun 14. Mol Psychiatry. 2022. PMID: 35701597
References
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. - PubMed
- Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. - PubMed
- Araneda R, Andrade R. 5-Hydroxytryptamine 2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. - PubMed
- Ashby CR, Jr, Edwards E, Harkins K, Wang RY. Effects of (+/−)-DOI on medial prefrontal cortical cells: a microiontophoretic study. Brain Res. 1989;498:393–396. - PubMed
- Astman N, Gutnick MJ, Fleidervish IA. Activation of protein kinase C increases neuronal excitability by regulating persistent Na+ current in mouse neocortical slices. J Neurophysiol. 1998;80:1547–1551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 DA006089/DA/NIDA NIH HHS/United States
- R01 MH062070/MH/NIMH NIH HHS/United States
- NS35180/NS/NINDS NIH HHS/United States
- MH62070/MH/NIMH NIH HHS/United States
- R01 NS035180/NS/NINDS NIH HHS/United States
- F32 MH064322/MH/NIMH NIH HHS/United States
- MH64322/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous