Complex gangliosides at the neuromuscular junction are membrane receptors for autoantibodies and botulinum neurotoxin but redundant for normal synaptic function - PubMed (original) (raw)
Complex gangliosides at the neuromuscular junction are membrane receptors for autoantibodies and botulinum neurotoxin but redundant for normal synaptic function
Roland W M Bullens et al. J Neurosci. 2002.
Abstract
One specialization of vertebrate presynaptic neuronal membranes is their multifold enrichment in complex gangliosides, suggesting that these sialoglycolipids may play a major functional role in synaptic transmission. We tested this hypothesis directly by studying neuromuscular synapses of mice lacking complex gangliosides attributable to deletion of the gene coding for beta1,4 GalNAc-transferase (GM2/GD2 synthase), which catalyzes an early step in ganglioside synthesis. Our studies show that complex gangliosides are surprisingly redundant for regulated neurotransmitter release under normal physiological conditions. In contrast, we show that they are membrane receptors for both the paralytic botulinum neurotoxin type-A and human neuropathy-associated anti-ganglioside autoantibodies that arise through molecular mimicry with microbial structures. These data prove the critical importance of complex gangliosides in mediating pathophysiological events at the neuromuscular synapse.
Figures
Fig. 1.
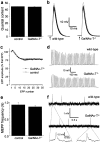
Ganglioside biosynthesis. In the investigated GalNAc-T−/− mice, disruption of the_N_-acetylgalactosaminyl-transferase (GalNAc-T) gene results in the absence of all the complex gangliosides within the dashed rectangle(Takamiya et al., 1996). Ganglioside nomenclature is according toSvennerholm (1994). CER, Ceramide;GalNAc, _N_-acetylgalactosamine;LacCer, lactosylceramide; NeuAc, neuraminic acid or sialic acid.
Fig. 2.
Basic synaptic electrophysiology at GalNAc-T−/− neuromuscular junctions. Measurements were performed at 20°C in diaphragms of six GalNAc-T−/− and six control (3 wild-type and 3 heterozygous) mice. No significant differences were observed in nerve stimulation-evoked and spontaneous ACh release. a, Quantal content of the endplate potential (EPP) at 0.3 Hz nerve stimulation. b, Typical examples of recorded EPPs; 30 consecutive EPPs at 0.3 Hz have been superimposed. The resting membrane potential during these measurements was found to be similar at wild-type and GalNAc-T−/− NMJs (−75.2 ± 0.5 and −74.6 ± 0.2 mV, respectively; means ± SEM;p = 0.24). The moment of nerve stimulation is indicated with an arrow. c, Average profiles of ACh release evoked by 40 Hz nerve stimulation.d, Typical examples of recorded EPP trains during 40 Hz stimulation. e, Spontaneous ACh release, measured as the frequency of miniature endplate potentials (MEPP), the spontaneous uniquantal events. f, Typical examples of recordings of MEPPs. No differences in amplitude or shape of MEPPs were observed. Error bars in a, c, and_e_ represent SE of the grand mean values in each group.
Fig. 3.
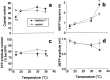
Temperature-dependency of electrophysiological synaptic parameters at GalNAc-T−/− neuromuscular junctions. Each temperature group consisted of four to six diaphragms. Controls were wild-type mice, except from the 20°C group that consisted of three wild-type and three heterozygous muscles.a, A reduction of 29% (p < 0.01) in low-rate (0.3 Hz) evoked ACh release was observed at 17°C at GalNAc-T−/− NMJs, compared with the control value at that temperature. b, Spontaneous ACh release was measured as the frequency of MEPPs, the spontaneous uniquantal events. There was a statistically significant increase (49%;p < 0.05) of spontaneous release at 35°C in GalNAc-T−/− NMJs, compared with control. At 17°C, a slight decrease (19%; p < 0.05) was observed. c, Rundown of ACh release during tetanic stimulation (40 Hz at 20°C, 33 Hz at the other temperatures). The rundown is given as the average value of the 21st–35th endplate potential (EPP), which forms the plateau phase of the train, and expressed as the percentage of the first EPP in a train. Slightly larger rundown was observed at GalNAc-T−/− NMJs at 30 and 35°C, compared with control. d, The amplitude of MEPPs did not differ statistically significantly between the genotypes at the different temperatures (*p < 0.05; **p< 0.01).
Fig. 4.
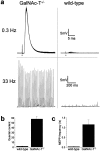
Botulinum neurotoxin lacks effect on ACh release at GalNAc-T−/− neuromuscular junctions. Three GalNAc-T−/− and three wild-type diaphragms were treated with 50 U/ml (∼2 ng/ml) botulinum neurotoxin type-A. The resulting effects on ACh release at NMJs were recorded electrophysiologically. At wild-type NMJs the toxin greatly depressed low-rate (0.3 Hz) and high-rate (33 Hz) nerve stimulation-evoked ACh release, as well as the spontaneous release. GalNAc-T−/− NMJs were resistant to this action of the toxin. a, Typical examples of 0.3 and 33 Hz evoked EPPs at botulinum neurotoxin-treated GalNAc-T−/−and wild-type NMJs. Thirty 0.3 Hz EPPs have been superimposed. The moment of nerve stimulation is indicated with a black dot. Note the dramatic decrease in EPP amplitude and the occurrence of failures at wild-type NMJs at both 0.3 and 33 Hz stimulation frequency. b, Average ± SEM of quantal content of low-rate evoked EPPs. c, Average ± SEM of spontaneous ACh release, measured as uniquantal miniature EPP (MEPP) frequency. Note that values of the GalNAc-T−/− NMJ parameters are similar to those found in earlier series without toxin treatment (Fig. 2), indicating that there was not a partial effect of the botulinum neurotoxin at this concentration at GalNAc-T−/− NMJs.
Fig. 5.
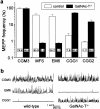
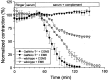
Induction of the α-Latrotoxin-like effects at neuromuscular junctions of GalNAc-T−/− and control mice by anti-ganglioside mouse monoclonal antibodies and Miller Fisher syndrome serum. Spontaneous ACh release, measured as frequency of miniature endplate potentials (MEPP), was recorded in four to six hemi-diaphragms that were pretreated with 50 μg/ml of the mAb or complement-inactivated Miller Fisher syndrome (MFS) serum (1:2), except for the CGG1 groups that each consisted of three muscles (control group: 2 heterozygous and 1 wild-type mouse). The measurements were done in the presence of normal human serum (1:2) as a source of complement, as described in Materials and Methods. The anti-GQ1b/GD3 specificities of the mAbs used are given in Table 1. a, Average values ± SEM of the MEPP frequencies. The monospecific anti-GQ1b mAb EM6 and the MFS serum induced the αLTx-like effect at wild-type NMJs but not at GalNAc-T−/− NMJs. Conversely, the monospecific anti-GD3 mAbs CGG1 and CGG2 induced the effect at GalNAc-T−/− but not at control NMJs. The bispecific anti-GQ1b/GD3 mAb CGM3 induced the effect at NMJs of all genotypes. The numbers in the _bars_represent the percentage of NMJs that displayed a MEPP frequency higher than four times the mean of the control value obtained before the mAb or MFS serum incubation (data not shown). b, Typical examples of electrophysiological recordings of MEPPs at NMJs treated with the prototype mAb CGM3, EM6, or CGG1.
Fig. 6.
Quantification of the paralysis induced by mono- and multispecific anti-GQ1b mAbs CGM3 and EM6 at GalNAc-T−/− and wild-type muscles. Contraction measurements were done in duplicate on GalNAc-T−/−and wild-type muscles pretreated with 50 μg/ml CGM3 or EM6 mAb. The contraction resulting from tetanic nerve stimulation (2 sec, 40 Hz) was recorded every 5 min. Before addition of complement-containing normal serum (1:2), contractions were measured during a 25 min period in Ringer's medium and a 20 min period in complement-inactivated normal serum (1:2). Symbols represent the peak of the tetanic contraction, expressed as the percentage of the average of the four peak values of the contractions recorded during the incubation period with complement-inactivated serum. The anti-GQ1b/GD3 mAb CGM3 completely paralyzed the muscles from both genotypes, although with a somewhat faster time course in GalNAc-T−/−muscles. The monospecific anti-GQ1b mAb EM6 induced partial paralysis (∼40%) in the wild-type muscles and was ineffective in the GalNAc-T−/− muscles.
Fig. 7.
Immunohistological analysis of deposition of complement and antibodies at neuromuscular junctions in GalNAc-T−/− and control muscle preparations treated with MFS serum and mouse monoclonal anti-GQ1b/GD3 antibodies.a, Typical examples of pictures taken of deposits of complement and antibody at NMJs in wild-type and GalNAc-T−/− preparations that had been treated with monospecific anti-GD3 mAb CGG2 and monospecific anti-GQ1b mAb EM6 (50 μg/ml) and had been studied electrophysiologically (Fig. 5). Shown are NMJs that were double stained with fluorescent α-BTx (top row in each panel) and fluorescent antibody against C3c or IgG (bottom row). Scale bars, 10 μm. BTx, α-Bungarotoxin;C3c, complement factor 3c; IgG, immunoglobulin G. b, Quantitative image analysis indicated that large complement deposits were present only in the samples where the αLatrotoxin-like effect at NMJs had been demonstrated electrophysiologically. As internal standards in the complement-staining quantification, the signal of CGM3-treated control NMJs was used in panel 1 and that of EM6-treated control NMJs was used in panel 2. Qualitative assessment of immunoglobulin showed colocalization of antibody and complement deposition at the NMJs in which the αLatrotoxin-like effect had occurred, except for the monospecific anti-GQ1b mAB EM6, which we could not demonstrate. Apparently, EM6 was deposited in amounts that were not detectable with our immunohistological methods. wt, Wild type; het, heterozygous; null, null-mutant; +, present; −, not present;nd, not determined.
Similar articles
- Roles of complex gangliosides at the neuromuscular junction.
Bullens RW, O'Hanlon GM, Wagner E, Molenaar PC, Furukawa K, Furukawa K, Plomp JJ, Willison HJ. Bullens RW, et al. Ann N Y Acad Sci. 2003 Sep;998:401-3. doi: 10.1196/annals.1254.051. Ann N Y Acad Sci. 2003. PMID: 14592906 No abstract available. - Neuromuscular synaptic function in mice lacking major subsets of gangliosides.
Zitman FM, Todorov B, Jacobs BC, Verschuuren JJ, Furukawa K, Furukawa K, Willison HJ, Plomp JJ. Zitman FM, et al. Neuroscience. 2008 Oct 28;156(4):885-97. doi: 10.1016/j.neuroscience.2008.08.034. Epub 2008 Aug 23. Neuroscience. 2008. PMID: 18801416 - Neuromuscular synaptic transmission in aged ganglioside-deficient mice.
Zitman FM, Todorov B, Verschuuren JJ, Jacobs BC, Furukawa K, Furukawa K, Willison HJ, Plomp JJ. Zitman FM, et al. Neurobiol Aging. 2011 Jan;32(1):157-67. doi: 10.1016/j.neurobiolaging.2009.01.007. Epub 2009 Feb 23. Neurobiol Aging. 2011. PMID: 19233512 - Complex gangliosides as autoantibody targets at the neuromuscular junction in Miller Fisher syndrome: a current perspective.
O'Hanlon GM, Bullens RW, Plomp JJ, Willison HJ. O'Hanlon GM, et al. Neurochem Res. 2002 Aug;27(7-8):697-709. doi: 10.1023/a:1020284302718. Neurochem Res. 2002. PMID: 12374204 Review. - Pathophysiological actions of neuropathy-related anti-ganglioside antibodies at the neuromuscular junction.
Plomp JJ, Willison HJ. Plomp JJ, et al. J Physiol. 2009 Aug 15;587(Pt 16):3979-99. doi: 10.1113/jphysiol.2009.171702. Epub 2009 Jun 29. J Physiol. 2009. PMID: 19564393 Free PMC article. Review.
Cited by
- Antiganglioside antibodies and paraneoplastic neuromuscular junction disorder?
Jazebi N, Patel C, Fang X. Jazebi N, et al. eNeurologicalSci. 2020 Oct 5;21:100277. doi: 10.1016/j.ensci.2020.100277. eCollection 2020 Dec. eNeurologicalSci. 2020. PMID: 33072897 Free PMC article. - Botulinum Toxin Injection-Site Selection for a Smooth Shoulder Line: An Anatomical Study.
Lee JH, Lee KY, Kim JY, Son WH, Jeong JH, Gil Jeong Y, Kwon S, Han SH. Lee JH, et al. Biomed Res Int. 2017;2017:3092720. doi: 10.1155/2017/3092720. Epub 2017 Jan 26. Biomed Res Int. 2017. PMID: 28246594 Free PMC article. - Receptor and substrate interactions of clostridial neurotoxins.
Brunger AT, Rummel A. Brunger AT, et al. Toxicon. 2009 Oct;54(5):550-60. doi: 10.1016/j.toxicon.2008.12.027. Epub 2009 Mar 4. Toxicon. 2009. PMID: 19268493 Free PMC article. Review. - Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology.
Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Pirazzini M, et al. Pharmacol Rev. 2017 Apr;69(2):200-235. doi: 10.1124/pr.116.012658. Pharmacol Rev. 2017. PMID: 28356439 Free PMC article. Review. - Distinct contributions of Galgt1 and Galgt2 to carbohydrate expression and function at the mouse neuromuscular junction.
Singhal N, Xu R, Martin PT. Singhal N, et al. Mol Cell Neurosci. 2012 Nov;51(3-4):112-26. doi: 10.1016/j.mcn.2012.08.014. Epub 2012 Sep 7. Mol Cell Neurosci. 2012. PMID: 22982027 Free PMC article.
References
- Ahnert-Hilger G, Bigalke H. Molecular aspects of tetanus and botulinum neurotoxin poisoning. Prog Neurobiol. 1995;46:83–96. - PubMed
- Ando S, Tanaka Y, Waki H, Kon K, Iwamoto M, Fukui F. Gangliosides and sialylcholesterol as modulators of synaptic functions. Ann NY Acad Sci. 1998;845:232–239. - PubMed
- Bakry N, Kamata Y, Simpson LL. Lectins from Triticum vulgaris and Limax flavus are universal antagonists of botulinum neurotoxin and tetanus toxin. J Pharmacol Exp Ther. 1991;258:830–836. - PubMed
- Bigalke H, Muller H, Dreyer F. Botulinum A neurotoxin unlike tetanus toxin acts via a neuraminidase sensitive structure. Toxicon. 1986;24:1065–1074. - PubMed
- Buchwald B, Weishaupt A, Toyka KV, Dudel J. Pre- and postsynaptic blockade of neuromuscular transmission by Miller-Fisher syndrome IgG at mouse motor nerve terminals. Eur J Neurosci. 1998;10:281–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases