Identification of domains of netrin UNC-6 that mediate attractive and repulsive guidance and responses from cells and growth cones - PubMed (original) (raw)
Comparative Study
Identification of domains of netrin UNC-6 that mediate attractive and repulsive guidance and responses from cells and growth cones
Yoo-shick Lim et al. J Neurosci. 2002.
Abstract
Netrin UNC-6 is a protein secreted from ventral cells that guides cell and growth cone migrations in Caenorhabditis elegans. Previously it was shown that UNC-6 domain V-2 regulates dorsal guidance activity and domain C regulates an activity that prevents the branching of axons when they respond to the N-terminal domains. Because these results indicate that the biological activities of UNC-6 are mediated through specific domains, we systematically examined each UNC-6 domain for guidance activities. Transgenic animals expressing UNC-6 derivatives with domain deletions and mutants with selective unc-6 loss-of-function mutations were analyzed. The results indicate that the VI, V-2, and V-3 domains are primarily required for dorsal migrations and the VI and V-3 domains are required for ventral migrations. These domains are likely important for responses mediated by the UNC-5 and UNC-40 receptors, respectively. Deletion of V-3 and a V-3 point mutation selectively affect either cell or growth cone migrations, indicating that each migration requires unique interactions with UNC-6. Deletion of domain VI or of a conserved eight amino acid motif within VI causes loss of all UNC-6 guidance activities, and mutations within domain VI selectively affect different guidance activities, suggesting that domain VI regulates each response to UNC-6. We propose that individual UNC-6 domains mediate different signals, which act in parallel to regulate the morphological changes necessary for guidance.
Figures
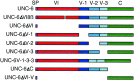
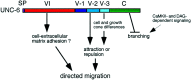
Fig. 1.
Schematic representation of the netrin UNC-6 protein and UNC-6 derivatives used in this study. UNC-6 comprises an N-terminal domain VI (residues 1–268) similar to the N-terminal domain VI of laminin subunits, three cysteine-rich repeats (residues 269–437) similar to those of domain V of laminin subunits, and a C-terminal domain named C (residues 438–591) that is not found in laminins but is phylogenetically conserved among other extracellular proteins. An epitope tag comprising three tandem copies of the HA epitope was engineered into a site immediately after the predicted signal peptide (SP).
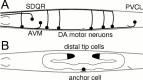
Fig. 2.
Summary of cell and axon positions.A, Cell bodies and axons of representative sensory and motor neurons. The migration of the axons of DA and DB motor neurons and of SDQR were assayed to measure the ability of _unc-6_mutations and transgenes to guide dorsal axon migrations. Only DA motor neurons are represented for simplicity. PVCL and AVM axons were assayed to measure ventral axon migrations. B, Migrating mesodermal cells, the distal tip cells, and the anchor cell were assayed to measure dorsal and ventral cell migrations, respectively. Details of the phenotypes of these cells in unc-5, unc-6, and unc-40 mutants were reported byHedgecock et al. (1990). Anterior is to the left; dorsal is at top.
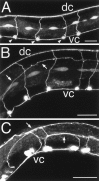
Fig. 3.
Dorsal axon migrations of DA and DB motor neurons.A, In wild-type animals, the DA and DB cell bodies (arrowheads) are positioned along the ventral nerve cord, and each has an axon that migrates longitudinally along the ventral nerve cord (vc) and an axon that migrates circumferentially to the dorsal nerve cord (dc). B, In some mutants, the dorsal circumferential migration defects are relatively mild. In this_unc-40_ mutant, a single axon (arrows) has abnormally migrated at the dorsal sublateral position and fails to reach the dorsal nerve cord. C, In unc-6_mutants, the axons rarely reach the dorsal nerve cord. In this_unc-6 mutant, most axons turn and migrate at the ventral sublateral and lateral positions (arrows). Anterior is to the left; dorsal is at top. Scale bars, 25 μm.
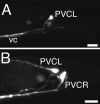
Fig. 4.
Ventral axon migrations of PVC in the tail region.A, In wild-type animals, the PVC neurons extend an axon ventrally along the lumbar commissure to the ventral nerve cord (vc). The left PVC neuron (PVCL) is shown. B, In unc-6 mutants, the PVC axons often migrate at the lateral position instead of directly entering the ventral nerve cord. In this animal, the axon from the left PVC neuron (PVCL) has migrated laterally, whereas the axon from the right PVC neuron (PVCR) has correctly migrated to the ventral nerve cord. Anterior is to the left; dorsal is at top. Scale bars, 10 μm.
Fig. 5.
A summary of the proposed function for each of the UNC-6 domains. The combination of domains elicits parallel signals that together mediate the different cytoskeletal changes necessary for guidance.
Similar articles
- Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans.
Wadsworth WG, Bhatt H, Hedgecock EM. Wadsworth WG, et al. Neuron. 1996 Jan;16(1):35-46. doi: 10.1016/s0896-6273(00)80021-5. Neuron. 1996. PMID: 8562088 - Moving around in a worm: netrin UNC-6 and circumferential axon guidance in C. elegans.
Wadsworth WG. Wadsworth WG. Trends Neurosci. 2002 Aug;25(8):423-9. doi: 10.1016/s0166-2236(02)02206-3. Trends Neurosci. 2002. PMID: 12127760 Review. - The C domain of netrin UNC-6 silences calcium/calmodulin-dependent protein kinase- and diacylglycerol-dependent axon branching in Caenorhabditis elegans.
Wang Q, Wadsworth WG. Wang Q, et al. J Neurosci. 2002 Mar 15;22(6):2274-82. doi: 10.1523/JNEUROSCI.22-06-02274.2002. J Neurosci. 2002. PMID: 11896167 Free PMC article. - SDQR migrations in Caenorhabditis elegans are controlled by multiple guidance cues and changing responses to netrin UNC-6.
Kim S, Ren XC, Fox E, Wadsworth WG. Kim S, et al. Development. 1999 Sep;126(17):3881-90. doi: 10.1242/dev.126.17.3881. Development. 1999. PMID: 10433916 - Genetic analysis of growth cone migrations in Caenorhabditis elegans.
Merz DC, Culotti JG. Merz DC, et al. J Neurobiol. 2000 Aug;44(2):281-8. J Neurobiol. 2000. PMID: 10934329 Review.
Cited by
- A Genetic Model of the Connectome.
Barabási DL, Barabási AL. Barabási DL, et al. Neuron. 2020 Feb 5;105(3):435-445.e5. doi: 10.1016/j.neuron.2019.10.031. Epub 2019 Dec 2. Neuron. 2020. PMID: 31806491 Free PMC article. - Phenotypic analysis of mice completely lacking netrin 1.
Yung AR, Nishitani AM, Goodrich LV. Yung AR, et al. Development. 2015 Nov 1;142(21):3686-91. doi: 10.1242/dev.128942. Epub 2015 Sep 22. Development. 2015. PMID: 26395479 Free PMC article. - Uncovering the genetic blueprint of the C. elegans nervous system.
Kovács IA, Barabási DL, Barabási AL. Kovács IA, et al. Proc Natl Acad Sci U S A. 2020 Dec 29;117(52):33570-33577. doi: 10.1073/pnas.2009093117. Epub 2020 Dec 14. Proc Natl Acad Sci U S A. 2020. PMID: 33318182 Free PMC article. - Potential therapeutics for myocardial ischemia-reperfusion injury. Focus on "Induction of cardioprotection by small netrin-1-derived peptides".
Cui MZ. Cui MZ. Am J Physiol Cell Physiol. 2015 Jul 15;309(2):C97-9. doi: 10.1152/ajpcell.00150.2015. Epub 2015 Jun 3. Am J Physiol Cell Physiol. 2015. PMID: 26040896 Free PMC article. No abstract available. - Solution Structure of C. elegans UNC-6: A Nematode Paralogue of the Axon Guidance Protein Netrin-1.
Krahn N, Meier M, Reuten R, Koch M, Stetefeld J, Patel TR. Krahn N, et al. Biophys J. 2019 Jun 4;116(11):2121-2130. doi: 10.1016/j.bpj.2019.04.033. Epub 2019 May 3. Biophys J. 2019. PMID: 31103237 Free PMC article.
References
- Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386:838–842. - PubMed
- Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. - PubMed
- Colavita A, Krishna S, Zheng H, Padgett RW, Culotti JG. Pioneer axon guidance by UNC-129, a C. elegans TGF-beta. Science. 1998;281:706–709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources