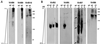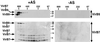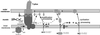Detergent extraction identifies different VirB protein subassemblies of the type IV secretion machinery in the membranes of Agrobacterium tumefaciens - PubMed (original) (raw)
Detergent extraction identifies different VirB protein subassemblies of the type IV secretion machinery in the membranes of Agrobacterium tumefaciens
Lilian Krall et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The VirB/D4 type IV secretion system of Agrobacterium tumefaciens translocates virulence factors (VirE2, VirF, and the VirD2-T-DNA complex) to plant cells. The membrane-bound translocation machinery consists of 12 proteins (VirB1-11 and VirD4) required for substrate translocation. Protein-protein interactions in the membranes were analyzed after extraction with the mild detergent dodecyl-beta-d-maltoside followed by separation under native conditions. Incubation of the membranes with increasing concentrations of the detergent differentially extracted virulence proteins. Separation of the solubilized proteins by blue native electrophoresis revealed cofractionation between two classes of protein complexes containing VirB7. The first class, consisting of major T-pilus component VirB2 and associated proteins VirB5 and VirB7, comigrated in the low molecular mass portion of the gel of about 100 kDa. The second class contains putative translocation complex core components VirB8, VirB9, and VirB10 in the high molecular mass portion of the gel larger than 232 kDa, as well as VirB7. Solubilized proteins were characterized further by gel filtration chromatography. This procedure separated T-pilus-associated proteins VirB2, VirB5, and VirB7 in the low molecular mass range from the other components of the translocation machinery and the substrates VirE2 and VirD2. Fractionation of VirB7-containing complexes (VirB7-VirB7 homodimers and VirB7-VirB9 heterodimers) suggested that they may link the T-pilus components to the core of the translocation machinery. Based on previously described VirB protein interactions and biochemical analysis of C58 wild type as well as of virB5 and virB6 deletion mutants, a model of T-pilus assembly in A. tumefaciens is suggested.
Figures
Fig 1.
DDM differentially extracts virulence proteins from the membranes. Virulence gene-induced (+AS) and uninduced C58 cells (−AS) were lysed, followed by fractionation of the total cell lysate (T) into soluble (S) and membrane fractions (M). Membranes were incubated with different concentrations of DDM; soluble proteins (S) were separated by ultracentrifugation from insoluble proteins (P). The different fractions were analyzed by SDS/PAGE and Western blotting with specific antisera. Molecular masses of reference proteins are shown on the right.
Fig 2.
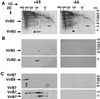
Blue native gel electrophoresis separates virulence protein complexes of different molecular masses. Membranes from virulence gene-induced (+AS) and uninduced cells (−AS; C58, wt; CB1005, Δ5; CB1006, Δ6) were incubated with 2% (wt/vol) DDM, and soluble proteins were mixed with Coomassie blue G-250. Separation was achieved by electrophoresis in native acrylamide gradient gels, followed by Western blotting with specific antisera. VirB7-specific signals in different molecular mass ranges are indicated by arrows. Molecular masses of reference proteins are shown on the right.
Fig 3.
Second-dimension SDS/PAGE resolves virulence proteins from complexes of the first-dimension native gel. Lanes containing DDM-extracted proteins from virulence gene-induced (+AS) and uninduced strain C58 (−AS) resolved by first-dimension blue native PAGE (1 D) were excised from the gel and incubated in Laemmli sample buffer. Lanes were applied on top of a 12% tricine gel, and proteins were separated by electrophoresis (2 D), followed by silver staining (A). Alternatively, proteins were transferred to poly(vinylidene difluoride) membranes followed by detection with VirB2- and VirB5-specific (B), or VirB7- and VirB9-specific antisera (C). Arrowhead indicates VirB2 in silver-stained gel. Molecular masses of reference proteins are shown on the right.
Fig 4.
Gel filtration chromatography separates virulence protein complexes of different molecular masses. Membranes from virulence gene-induced (+AS) and uninduced strain C58 (−AS) were incubated with 2% DDM, and solubilized proteins were applied to a Superdex 200 column. Eluted proteins were collected by TCA precipitation, subjected to SDS/PAGE under reducing conditions, followed by Western blotting with specific antisera. (A) Proteins eluting in high molecular mass complexes. (B) Proteins eluting in low molecular mass complexes. Higher molecular mass forms of VirB2 are indicated by an arrowhead. Molecular masses of reference proteins are shown on the right.
Fig 5.
Analysis of complexes of VirB7 and VirB9 under nonreducing conditions. Membranes from virulence gene-induced (+AS) and uninduced strain C58 (−AS) were extracted with 2% (wt/vol) DDM, and solubilized proteins were applied to chromatography on a Superdex 200 column. Eluted proteins were collected by TCA precipitation, subjected to SDS/PAGE under nonreducing conditions, followed by Western blotting with specific antisera. Molecular masses of reference proteins are shown on the right.
Fig 6.
Hypothetical model for the mechanism of T-pilus assembly. After removal of signal peptides from VirB2 and VirB5 and cyclization of VirB2, the major T-pilus component associates with VirB5 in the membranes. VirB6 is essential for stabilization of VirB5, and this effect may rely on direct binding or necessitate other proteins. N-terminal lipoprotein processing of VirB7 is followed by VirB6-assisted formation of VirB7 homodimers, which may link the T-pilus to the core complex. Energy for T-pilus assembly may be provided by VirB11 (20), and it may be transduced to the pilus assembly complex in the outer membrane via VirB6 and VirB7. SPI and SPII, signal peptidases.
Similar articles
- Agrobacterium tumefaciens VirB6 protein participates in formation of VirB7 and VirB9 complexes required for type IV secretion.
Jakubowski SJ, Krishnamoorthy V, Christie PJ. Jakubowski SJ, et al. J Bacteriol. 2003 May;185(9):2867-78. doi: 10.1128/JB.185.9.2867-2878.2003. J Bacteriol. 2003. PMID: 12700266 Free PMC article. - Elevated temperature differentially affects virulence, VirB protein accumulation, and T-pilus formation in different Agrobacterium tumefaciens and Agrobacterium vitis strains.
Baron C, Domke N, Beinhofer M, Hapfelmeier S. Baron C, et al. J Bacteriol. 2001 Dec;183(23):6852-61. doi: 10.1128/JB.183.23.6852-6861.2001. J Bacteriol. 2001. PMID: 11698374 Free PMC article. - VirB7 lipoprotein is exocellular and associates with the Agrobacterium tumefaciens T pilus.
Sagulenko V, Sagulenko E, Jakubowski S, Spudich E, Christie PJ. Sagulenko V, et al. J Bacteriol. 2001 Jun;183(12):3642-51. doi: 10.1128/JB.183.12.3642-3651.2001. J Bacteriol. 2001. PMID: 11371529 Free PMC article. - Assembly of the VirB transport complex for DNA transfer from Agrobacterium tumefaciens to plant cells.
Zupan JR, Ward D, Zambryski P. Zupan JR, et al. Curr Opin Microbiol. 1998 Dec;1(6):649-55. doi: 10.1016/s1369-5274(98)80110-0. Curr Opin Microbiol. 1998. PMID: 10066547 Review. - VirB8: a conserved type IV secretion system assembly factor and drug target.
Baron C. Baron C. Biochem Cell Biol. 2006 Dec;84(6):890-9. doi: 10.1139/o06-148. Biochem Cell Biol. 2006. PMID: 17215876 Review.
Cited by
- Plant proteins that interact with VirB2, the Agrobacterium tumefaciens pilin protein, mediate plant transformation.
Hwang HH, Gelvin SB. Hwang HH, et al. Plant Cell. 2004 Nov;16(11):3148-67. doi: 10.1105/tpc.104.026476. Epub 2004 Oct 19. Plant Cell. 2004. PMID: 15494553 Free PMC article. - Small heat-shock protein HspL is induced by VirB protein(s) and promotes VirB/D4-mediated DNA transfer in Agrobacterium tumefaciens.
Tsai YL, Wang MH, Gao C, Klüsener S, Baron C, Narberhaus F, Lai EM. Tsai YL, et al. Microbiology (Reading). 2009 Oct;155(Pt 10):3270-3280. doi: 10.1099/mic.0.030676-0. Epub 2009 Jun 25. Microbiology (Reading). 2009. PMID: 19556291 Free PMC article. - Immunogenicity of Anaplasma marginale type IV secretion system proteins in a protective outer membrane vaccine.
Lopez JE, Palmer GH, Brayton KA, Dark MJ, Leach SE, Brown WC. Lopez JE, et al. Infect Immun. 2007 May;75(5):2333-42. doi: 10.1128/IAI.00061-07. Epub 2007 Mar 5. Infect Immun. 2007. PMID: 17339347 Free PMC article. - VirB1 orthologs from Brucella suis and pKM101 complement defects of the lytic transglycosylase required for efficient type IV secretion from Agrobacterium tumefaciens.
Höppner C, Liu Z, Domke N, Binns AN, Baron C. Höppner C, et al. J Bacteriol. 2004 Mar;186(5):1415-22. doi: 10.1128/JB.186.5.1415-1422.2004. J Bacteriol. 2004. PMID: 14973016 Free PMC article. - Blue-native PAGE in plants: a tool in analysis of protein-protein interactions.
Eubel H, Braun HP, Millar AH. Eubel H, et al. Plant Methods. 2005 Nov 16;1(1):11. doi: 10.1186/1746-4811-1-11. Plant Methods. 2005. PMID: 16287510 Free PMC article.
References
- Lessl M. & Lanka, E. (1994) Cell 77, 321-324. - PubMed
- Salmond G. P. C. (1994) Annu. Rev. Phytopathol. 32, 181-200.
- Covacci A., Telford, J. L., Del Giudice, G., Parsonnet, J. & Rappuoli, R. (1999) Science 284, 1328-1333. - PubMed
- Baron C., O'Callaghan, D. & Lanka, E. (2002) Mol. Microbiol. 43, 1359-1366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials