Salmonella enterica serovar Typhimurium periplasmic superoxide dismutases SodCI and SodCII are required for protection against the phagocyte oxidative burst - PubMed (original) (raw)
Salmonella enterica serovar Typhimurium periplasmic superoxide dismutases SodCI and SodCII are required for protection against the phagocyte oxidative burst
Laura M Sly et al. Infect Immun. 2002 Sep.
Abstract
Vitamin D(3) (1,25-dihydroxycholecalciferol) induced the phagocyte oxidative burst and intracellular killing of Salmonella enterica serovar Typhimurium in a phosphatidylinositol 3-kinase-dependent manner. The antimicrobial effect was more pronounced for Salmonella SodCI and SodCII mutants, confirming the role of the phagocyte oxidase in the vitamin D(3) effect. The results for an in vitro system with human THP-1 cells correlate with in vivo virulence data for mice and show that both the SodCI and SodCII enzymes are required to protect against the oxidative burst.
Figures
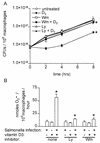
FIG. 1.
Vitamin D3 restricts the intracellular growth of wild-type S. enterica serovar Typhimurium and induces the phagocyte oxidative burst in a PI 3-kinase-dependent manner. (A) Intracellular S. enterica serovar Typhimurium quantitated by serial dilution in Luria-Bertani broth and plating on Luria-Bertani agar 0, 2, 4, and 8 h after infection of THP-1 cells. Cells were either not treated or treated with the PI 3-K inhibitor Wm (50 nM) or Ly (15 μM) and then were left untreated or treated with vitamin D3 (100 nM). The data are averages from three independent experiments performed in triplicate. An asterisk indicates that the P value was <0.03 when the growth in vitamin D3-treated cells was compared to the growth in control cells or when the growth in cells treated with Ly or Wm plus vitamin D3 was compared to the growth in cells treated only with vitamin D3, as determined by analysis of variance at each time point. (B) Superoxide secreted by macrophages in 1 h determined by measuring the Sod-inhibitable reduction of ferricytochrome c in the presence and absence of Salmonella infection, vitamin D3 treatment, and the inhibitor Ly or Wm. An asterisk indicates that the P value was <0.02 when the data for Salmonella infection and vitamin D3 treatment was compared to the data for either treatment alone or when the data for pretreatment with Ly or Wm followed by Salmonella infection and vitamin D3 treatment was compared to the data for no pretreatment, as determined by an unpaired Student's t test.
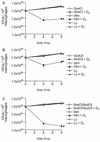
FIG. 2.
S. enterica serovar Typhimurium Sod mutants are more susceptible to the antimicrobial activity of vitamin D3 than the wild-type strain. Differentiated THP-1 cells were infected with wild-type S. enterica serovar Typhimuriu_m_ (wt) or with a SodCI, SodCII, or double SodCI-SodCII S. enterica serovar Typhimurium mutant and then were left untreated or were treated with vitamin D3. The CFU of intracellular bacteria were enumerated 0, 4, and 8 h after infection. The data are averages from three independent experiments performed in triplicate. An asterisk indicates that the P value was <0.05 when the growth of each mutant in vitamin D3-treated cells was compared to the growth in control cells, as determined by analysis of variance at each time point.
FIG. 3.
Killing of intracellular S. enterica serovar Typhimurium Sod mutants by vitamin D3 is PI 3-K dependent. Differentiated THP-1 cells were infected with the SodCI mutant (A), the SodCII mutant (B), and the SodCI-SodCII double mutant (C). Cells either were left untreated or were treated with Wm or Ly and then were either left untreated or treated with vitamin D3. The CFU of intracellular bacteria were enumerated 0, 4, and 8 h after infection. The data are from three independent experiments performed in triplicate. An asterisk indicates that the P value was <0.04 (A), <0.05 (B), or <0.01 (C) when growth in vitamin D3-treated cells was compared to growth in control cells or when the data for pretreatment with Ly or Wm followed by Salmonella infection and vitamin D3 treatment of cells was compared to the data for no pretreatment of cells. Comparisons were made by performing analysis of variance at each time point.
Similar articles
- Differences in enzymatic properties allow SodCI but not SodCII to contribute to virulence in Salmonella enterica serovar Typhimurium strain 14028.
Krishnakumar R, Craig M, Imlay JA, Slauch JM. Krishnakumar R, et al. J Bacteriol. 2004 Aug;186(16):5230-8. doi: 10.1128/JB.186.16.5230-5238.2004. J Bacteriol. 2004. PMID: 15292124 Free PMC article. - Differences in gene expression levels and in enzymatic qualities account for the uneven contribution of superoxide dismutases SodCI and SodCII to pathogenicity in Salmonella enterica.
Figueroa-Bossi N, Ammendola S, Bossi L. Figueroa-Bossi N, et al. Microbes Infect. 2006 May;8(6):1569-78. doi: 10.1016/j.micinf.2006.01.015. Epub 2006 Apr 18. Microbes Infect. 2006. PMID: 16697686 - Salmonella enterica serovar Typhimurium periplasmic superoxide dismutase SodCI is a member of the PhoPQ regulon and is induced in macrophages.
Golubeva YA, Slauch JM. Golubeva YA, et al. J Bacteriol. 2006 Nov;188(22):7853-61. doi: 10.1128/JB.00706-06. Epub 2006 Sep 15. J Bacteriol. 2006. PMID: 16980468 Free PMC article. - Responses to reactive oxygen intermediates and virulence of Salmonella typhimurium.
Janssen R, van der Straaten T, van Diepen A, van Dissel JT. Janssen R, et al. Microbes Infect. 2003 May;5(6):527-34. doi: 10.1016/s1286-4579(03)00069-8. Microbes Infect. 2003. PMID: 12758282 Review. - Salmonella evasion of the NADPH phagocyte oxidase.
Vazquez-Torres A, Fang FC. Vazquez-Torres A, et al. Microbes Infect. 2001 Nov-Dec;3(14-15):1313-20. doi: 10.1016/s1286-4579(01)01492-7. Microbes Infect. 2001. PMID: 11755420 Review.
Cited by
- Structural properties of periplasmic SodCI that correlate with virulence in Salmonella enterica serovar Typhimurium.
Krishnakumar R, Kim B, Mollo EA, Imlay JA, Slauch JM. Krishnakumar R, et al. J Bacteriol. 2007 Jun;189(12):4343-52. doi: 10.1128/JB.00010-07. Epub 2007 Apr 6. J Bacteriol. 2007. PMID: 17416645 Free PMC article. - Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice.
Stecher B, Hapfelmeier S, Müller C, Kremer M, Stallmach T, Hardt WD. Stecher B, et al. Infect Immun. 2004 Jul;72(7):4138-50. doi: 10.1128/IAI.72.7.4138-4150.2004. Infect Immun. 2004. PMID: 15213159 Free PMC article. - Characterization of Salmonella-induced cell death in human macrophage-like THP-1 cells.
Valle E, Guiney DG. Valle E, et al. Infect Immun. 2005 May;73(5):2835-40. doi: 10.1128/IAI.73.5.2835-2840.2005. Infect Immun. 2005. PMID: 15845488 Free PMC article. - New Strategies to Promote Macrophage Cholesterol Efflux.
Choi HY, Ruel I, Choi S, Genest J. Choi HY, et al. Front Cardiovasc Med. 2021 Dec 23;8:795868. doi: 10.3389/fcvm.2021.795868. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 35004908 Free PMC article. - The distinctive roles played by the superoxide dismutases of the extremophile Acinetobacter sp. Ver3.
Steimbrüch BA, Sartorio MG, Cortez N, Albanesi D, Lisa MN, Repizo GD. Steimbrüch BA, et al. Sci Rep. 2022 Mar 12;12(1):4321. doi: 10.1038/s41598-022-08052-z. Sci Rep. 2022. PMID: 35279679 Free PMC article.
References
- De Groote, M. A., U. A. Ochsner, M. U. Shiloh, C. Nathan, J. M. McCord, M. C. Dinauer, S. J. Libby, A. Vazquez-Torres, Y. Xu, and F. C. Fang. 1997. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. USA 94:13997-14001. - PMC - PubMed
- DeLeo, F. R., M. A. Jutila, and M. T. Quinn. 1996. Characterization of peptide diffusion into electropermeabilized neutrophils. J. Immunol. Methods 198:35-49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 DK035108/DK/NIDDK NIH HHS/United States
- AI32178/AI/NIAID NIH HHS/United States
- DK35108/DK/NIDDK NIH HHS/United States
- R01 AI032178/AI/NIAID NIH HHS/United States
- R56 AI032178/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources