Potent and specific inhibition of human immunodeficiency virus type 1 replication by RNA interference - PubMed (original) (raw)
Potent and specific inhibition of human immunodeficiency virus type 1 replication by RNA interference
Glen A Coburn et al. J Virol. 2002 Sep.
Abstract
Synthetic small interfering RNAs (siRNAs) have been shown to induce the degradation of specific mRNA targets in human cells by inducing RNA interference (RNAi). Here, we demonstrate that siRNA duplexes targeted against the essential Tat and Rev regulatory proteins encoded by human immunodeficiency virus type 1 (HIV-1) can specifically block Tat and Rev expression and function. More importantly, we show that these same siRNAs can effectively inhibit HIV-1 gene expression and replication in cell cultures, including those of human T-cell lines and primary lymphocytes. These observations demonstrate that RNAi can effectively block virus replication in human cells and raise the possibility that RNAi could provide an important innate protective response, particularly against viruses that express double-stranded RNAs as part of their replication cycle.
Figures
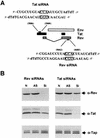
FIG. 1.
(A) Schematic representation of part of the HIV-1 genome. HIV-1 tat and rev exons are depicted with black and grey boxes, respectively, while the env open reading frame is shown as a white box (not to scale). The structure and target coordinates of the siRNAs specific for tat and rev are depicted. Boxed residues were mutated by transversion for use as control duplexes in some experiments. (B) siRNAs directed against tat and rev specifically inhibit Tat and Rev protein expression. Human 293T cells were cotransfected with 50 ng of both the pcTat and pcRev expression plasmid in the absence of RNA oligonucleotides (N) or in the presence of 0.1 μM antisense oligonucleotides (AS) or 0.1 μM siRNA duplexes (Si). Cell lysates were prepared 60 h posttransfection, and protein expression was assayed by Western analysis with polyclonal antiTat and antiRev antisera. Western blots were also probed with antibodies directed against the endogenous TAP/NXF-1 protein, which here served as an internal control for both loading and specificity.
FIG. 2.
(A) siRNAs directed against HIV-1 tat inhibit HIV-1 Tat but not BIV Tat function. Human 293T cells were transfected with 100 ng of pTAR/CAT or pbTAR/CAT, 50 ng of the internal control plasmid pBC12/CMV/β-Gal, and 0.1 ng of the indicated effector plasmid. siRNA oligonucleotides or siRNA duplexes were cotransfected with the reporter and expression plasmids as described in Materials and Methods. Cells were harvested 48 h after transfection, and induced CAT activities were determined. The data shown represent averages for three experiments with the standard deviations indicated by error bars. (B) siRNAs directed against HIV-1 rev specifically inhibit HIV-1 Rev but not HTLV Rex function. 293T cells were transfected with 25 ng of the pDM128/RRE or pDM128/RxRE reporter plasmid, 50 ng of pBC12/CMV/β-Gal, and 1 ng of the indicated effector plasmid. siRNA oligonucleotides or siRNA duplexes were cotransfected with the reporter and expression plasmids as described in Materials and Methods. Cells were harvested 48 h after transfection, and induced CAT and β-galactosidase activities were determined. The data shown represent averages for three experiments with the standard deviations indicated by error bars.
FIG. 3.
Inhibition of HIV-1 replication in human cells by RNAi. 293T cells were transfected with CD4 and CCR5 expression plasmids and siRNA duplexes (si) or antisense RNAs (as) (0.1 μM each). After 48 h, CD4+ CCR5+ cells were infected overnight with 50 ng of p24 antigen of the luciferase reporter virus NL-luc-ADA or the wild-type virus NL-ADA. Viral gene expression was determined by luciferase activity after 48 h of infection (A), while viral replication was measured by p24 Gag antigen production 60 h posttransfection (B). 293T cells lacking CD4 and CCR5 served as the negative control (NEG), while CD4+ CCR5+ 293T cells infected in the absence of siRNAs served as the positive control (POS). A low level of carryover of the virus stock used to infect the cells explains the background level of p24 Gag seen in panel B (vertical dashed line).
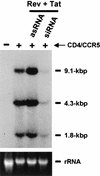
FIG. 4.
Analysis of HIV-1 RNA expression in human cells reveals that tat and rev siRNAs can reduce the expression of all three classes of HIV mRNA. Human 293T cells were cotransfected with siRNA duplexes (si) or with antisense RNAs (as) (0.1 μM each) and the CD4 and CCR5 expression plasmids as indicated at the top of the lanes. CD4+ CCR5+ cells were then infected overnight with 50 ng of p24 antigen. After 48 h of infection, total RNA was extracted from infected 293T cells and subjected to Northern analysis. The approximate sizes of the three classes of HIV-1 transcripts are indicated on the right. 18S rRNA, stained with ethidium bromide, is shown at the bottom of the figure as a loading control.
FIG. 5.
siRNAs directed against tat and rev can initiate the degradation of HIV-1 genomic RNA prior to reverse transcription. Human 293T cells were transfected with siRNA duplexes (si) or with antisense RNAs (as). CD4+ CCR5+ cells were infected overnight with 50 ng of p24 antigen. After 48 h, total DNA was extracted from infected 293T cells, denatured in 0.2 M NaOH, spotted onto a nylon membrane, and probed with a 32P-labeled HIV-1-specific probe as described in Materials and Methods. As a control for loading, equivalent amounts of DNA were probed with a 32P-labeled β-globin probe. 293T cells treated with 0.1 μM AZT, the reverse transcriptase inhibitor (2), are also shown (+AZT). DNA isolated from mock-infected 293T cells served as the negative control (NEG), while CD4+ CCR5+ 293T cells infected in the absence of siRNAs served as the positive control (POS).
FIG. 6.
siRNA inhibition of HIV-1 replication in T cells. (A) Jurkat T cells were transfected with a 12 μM concentration of each of the indicated siRNA duplex or single-stranded RNA oligonucleotides. Mock-transfected Jurkat T cells served as the positive control (POS), while the two 3-nt mismatch siRNAs shown in Fig. 1A served as the control (siRNA-M). After 24 h, transfected cells were infected overnight with VSV-G-pseudotyped NL-ADA. Viral production was determined by measurement of p24 Gag production at 48 h after infection. The data shown represent averages for three experiments with the standard deviations indicated by error bars. (B) Human PBMCs were transfected with the wild-type or mutant forms of the Tat and Rev siRNAs by electroporation and were infected with the R5-tropic NL-ADA strain 24 h later. Progeny virus production was measured 48 h after infection by p24 ELISA.
Similar articles
- Down-modulation of TCR/CD3 surface complexes after HIV-1 infection is associated with differential expression of the viral regulatory genes.
Willard-Gallo KE, Furtado M, Burny A, Wolinsky SM. Willard-Gallo KE, et al. Eur J Immunol. 2001 Apr;31(4):969-79. doi: 10.1002/1521-4141(200104)31:4<969::aid-immu969>3.0.co;2-2. Eur J Immunol. 2001. PMID: 11298321 - Antiviral effects of human immunodeficiency virus type 1-specific small interfering RNAs against targets conserved in select neurotropic viral strains.
Dave RS, Pomerantz RJ. Dave RS, et al. J Virol. 2004 Dec;78(24):13687-96. doi: 10.1128/JVI.78.24.13687-13696.2004. J Virol. 2004. PMID: 15564478 Free PMC article. - Protection of primary human T cells from HIV infection by Trev: a transdominant fusion gene.
Chinen J, Aguilar-Cordova E, Ng-Tang D, Lewis DE, Belmont JW. Chinen J, et al. Hum Gene Ther. 1997 May 1;8(7):861-8. doi: 10.1089/hum.1997.8.7-861. Hum Gene Ther. 1997. PMID: 9143912 - Inhibition of HIV-1 replication by targeting the Rev protein.
Nakaya T, Iwai S, Fujinaga K, Otsuka E, Ikuta K. Nakaya T, et al. Leukemia. 1997 Apr;11 Suppl 3:134-7. Leukemia. 1997. PMID: 9209321 Review. - Molecular biology of HIV-1: positive and negative regulatory elements important for virus expression.
Felber BK, Pavlakis GN. Felber BK, et al. AIDS. 1993;7 Suppl 1:S51-62. AIDS. 1993. PMID: 8363803 Review. No abstract available.
Cited by
- Antiviral Stratagems Against HIV-1 Using RNA Interference (RNAi) Technology.
Vlachakis D, Tsiliki G, Pavlopoulou A, Roubelakis MG, Tsaniras SC, Kossida S. Vlachakis D, et al. Evol Bioinform Online. 2013 May 16;9:203-13. doi: 10.4137/EBO.S11412. Print 2013. Evol Bioinform Online. 2013. PMID: 23761954 Free PMC article. - Inhibition of hepatitis B virus expression and replication by RNA interference in HepG2.2.15.
Zhao ZF, Yang H, Han DW, Zhao LF, Zhang GY, Zhang Y, Liu MS. Zhao ZF, et al. World J Gastroenterol. 2006 Oct 7;12(37):6046-9. doi: 10.3748/wjg.v12.i37.6046. World J Gastroenterol. 2006. PMID: 17009407 Free PMC article. - Inhibition of human immunodeficiency virus type 1 replication with artificial transcription factors targeting the highly conserved primer-binding site.
Eberhardy SR, Goncalves J, Coelho S, Segal DJ, Berkhout B, Barbas CF 3rd. Eberhardy SR, et al. J Virol. 2006 Mar;80(6):2873-83. doi: 10.1128/JVI.80.6.2873-2883.2006. J Virol. 2006. PMID: 16501096 Free PMC article. - Antiviral applications of RNAi.
Morris KV, Rossi JJ. Morris KV, et al. Handb Exp Pharmacol. 2006;173(173):105-16. doi: 10.1007/3-540-27262-3_6. Handb Exp Pharmacol. 2006. PMID: 16594613 Free PMC article. Review. - Biochemical prevention and treatment of viral infections - a new paradigm in medicine for infectious diseases.
Le Calvez H, Yu M, Fang F. Le Calvez H, et al. Virol J. 2004 Nov 23;1:12. doi: 10.1186/1743-422X-1-12. Virol J. 2004. PMID: 15560846 Free PMC article. Review.
References
- Ancellin, N., C. Colmont, J. Su, Q. Li, N. Mittereder, S. S. Chae, S. Steffansson, G. Liau, and T. Hla.2002. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J. Biol. Chem. 277:6667-6675. - PubMed
- Arts, E. J., M. E. Quiñones-Mateu, J. L. Albright, J.-P. Marois, C. Hough, Z. Gu, and M. A. Wainberg.1998. 3′-Azido-3′-deoxythymidine (AZT) mediates cross-resistance to nucleoside analogs in the case of AZT-resistant human immunodeficiency virus type 1 variants. J. Virol. 72:4858-4865. - PMC - PubMed
- Baltimore, D.1988. Intracellular immunization. Nature 335:395-396. - PubMed
- Bogerd, H. P., A. Echarri, T. M. Ross, and B. R. Cullen.1998. Inhibition of human immunodeficiency virus Rev and human T-cell leukemia virus Rex function, but not Mason-Pfizer monkey virus constitutive transport element activity, by a mutant human nucleoporin targeted to Crm1. J. Virol. 72:8627-8635. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources