Functional plasticity of an antigen-specific memory CD4 T cell population - PubMed (original) (raw)
Functional plasticity of an antigen-specific memory CD4 T cell population
Mojgan Ahmadzadeh et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The protective nature of memory immune responses is attributed largely to terminally differentiated memory T cells that retain memory of the antigen via the antigen receptor and memory of the effector functions that initially cleared the pathogen. It is not known whether a given population of antigen-specific memory T cells is endowed with functional flexibility to provide protective responses against antigens reencountered in different immunological contexts. Here, we examine functional properties of influenza hemagglutinin (HA)-specific memory CD4 T cells recovered from adoptive hosts that received in vitro-activated HA-specific T cell receptor-transgenic CD4 T cells 2 months to 1 year previously. We demonstrate that this HA-specific memory CD4 T cell population bearing a clonal T cell receptor can produce predominantly T helper 1 or T helper 2 effector cytokines depending on the nature of the recall stimulus. Our findings reveal remarkable functional plasticity within an antigen-specific memory T cell population and have direct implications for modulating memory T cell function in vaccine design and treatments for autoimmune diseases.
Figures
Figure 1
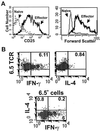
Phenotype and cytokine profile of HA-TCR effector CD4 T cells. (A) CD25 expression profile (Left) and size (forward scatter, Right) of 6.5+ HA-TCR naive and effector CD4 T cells. (B) Production of IFN-γ and IL-4 during generation of HA-TCR effector CD4 T cells. Intracellular cytokine analysis of IFN-γ (Upper Left) and IL-4 production (Upper Right) by 6.5+ HA-TCR effector CD4 T cells during their generation in vitro is shown. Monensin was added 2–3 days after activation of HA-TCR CD4 T cells with HA peptide and APC. IFN-γ versus IL-4 production is gated on 6.5+ cells (Lower).
Figure 2
Functional responses of differentially stimulated HA-TCR memory CD4 T cells. HA-TCR memory CD4 T cells were recovered 10 weeks and 1 year posttransfer of HA-TCR effector cells into RAG2−/− adoptive hosts. For controls, naive HA-TCR CD4 T cells were transferred into RAG2−/− hosts in parallel. Memory and naive CD4 T cells were restimulated with 1 μg/ml HA peptide (black bars) or 1 μg/ml anti-CD3 antibody (gray bars) in the presence of mitomycin C-treated APC. Freshly purified HA-TCR CD4 T cells (marked Fresh Tg or FTg) were cultured in parallel. IFN-γ and IL-4 content in 48-h culture supernatants was measured by specific ELISA, and proliferation was assessed by measuring [3H]thymidine incorporation after 72 h (see Materials and Methods). These results are representative of five different experiments.
Figure 3
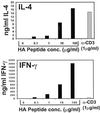
Cytokine production from HA-TCR memory CD4 T cells in response to increasing antigen doses. Memory CD4 T cells were isolated from adoptive hosts 6 months posttransfer and activated with the indicated doses of HA peptide or 1 μg/ml anti-CD3 antibody in the presence of APC. IFN-γ and IL-4 content in culture supernatants was quantitated by specific ELISA as described for Fig. 2.
Figure 4
ICS analysis of differentially stimulated naive and memory HA-TCR CD4 T cells. HA-TCR memory CD4 T cells isolated from adoptive hosts 3 months previously were reactivated with HA peptide (antigen) or anti-CD3 in the presence of APC followed by incubation with monensin, surface staining for 6.5 and CD25, and intracellular staining for IFN-γ, IL-4, and IL-2. (A) Production of IFN-γ from 6.5 TCR-expressing and CD25+ memory T cells gated on large, activated cells. Unactivated T cells showed no IFN-γ production, and quadrants are designated based on staining with isotype-matched control antibodies for each cytokine. Numbers in quadrants refer to the percentage of total activated cells. (B) Production of IL-4 from 6.5+ and CD25+ HA-TCR memory CD4 T cells. The numbers in parentheses refer to the absolute number of cells in each quadrant. Activation of naive HA-TCR CD4 T cells yielded negligible numbers of IL-4-producing cells (data not shown). (C) IL-4+ and IFN-γ+ memory T cells shown together. The numbers in parentheses indicates absolute cell number. (D) IL-2 and IFN-γ production from 6.5+ activated naive and memory CD4 T cells. The numbers in parentheses refer to the absolute numbers of 6.5+ T cells, and data shown are gated on 6.5+ CD4+ T cells.
Similar articles
- Antigen-specific CD4 T cell clonal expansion and differentiation in the aged lymphoid microenvironment. II. The memory T cell response is diminished.
Mittler JN, Lee WT. Mittler JN, et al. Mech Ageing Dev. 2004 Jan;125(1):59-68. doi: 10.1016/j.mad.2003.10.003. Mech Ageing Dev. 2004. PMID: 14706238 - The effector to memory transition of CD4 T cells.
McKinstry KK, Strutt TM, Swain SL. McKinstry KK, et al. Immunol Res. 2008;40(2):114-27. doi: 10.1007/s12026-007-8004-y. Immunol Res. 2008. PMID: 18213525 Review. - Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms.
Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. Teijaro JR, et al. J Virol. 2010 Sep;84(18):9217-26. doi: 10.1128/JVI.01069-10. Epub 2010 Jun 30. J Virol. 2010. PMID: 20592069 Free PMC article. - Low-dose antigen-experienced CD4+ T cells display reduced clonal expansion but facilitate an effective memory pool in response to secondary exposure.
Park SO, Han YW, Aleyas AG, George JA, Yoon HA, Lee JH, Kang HY, Kang SH, Eo SK. Park SO, et al. Immunology. 2008 Mar;123(3):426-37. doi: 10.1111/j.1365-2567.2007.02707.x. Epub 2007 Oct 4. Immunology. 2008. PMID: 17916164 Free PMC article. - Generation, persistence and plasticity of CD4 T-cell memories.
Lees JR, Farber DL. Lees JR, et al. Immunology. 2010 Aug;130(4):463-70. doi: 10.1111/j.1365-2567.2010.03288.x. Epub 2010 May 10. Immunology. 2010. PMID: 20465569 Free PMC article. Review.
Cited by
- T-helper cells flexibility: the possibility of reprogramming T cells fate.
Khantakova JN, Sennikov SV. Khantakova JN, et al. Front Immunol. 2023 Nov 1;14:1284178. doi: 10.3389/fimmu.2023.1284178. eCollection 2023. Front Immunol. 2023. PMID: 38022605 Free PMC article. Review. - Measuring the Manipulation of T Helper Immune Responses by Schistosoma mansoni.
Tedla MG, Every AL, Scheerlinck JY. Tedla MG, et al. Int J Mol Sci. 2022 Jan 27;23(3):1462. doi: 10.3390/ijms23031462. Int J Mol Sci. 2022. PMID: 35163381 Free PMC article. - Increased CD4+ T cell lineage commitment determined by CpG methylation correlates with better prognosis in urinary bladder cancer patients.
Ahlén Bergman E, Hartana CA, Johansson M, Linton LB, Berglund S, Hyllienmark M, Lundgren C, Holmström B, Palmqvist K, Hansson J, Alamdari F, Huge Y, Aljabery F, Riklund K, Winerdal ME, Krantz D, Zirakzadeh AA, Marits P, Sjöholm LK, Sherif A, Winqvist O. Ahlén Bergman E, et al. Clin Epigenetics. 2018 Aug 3;10(1):102. doi: 10.1186/s13148-018-0536-6. Clin Epigenetics. 2018. PMID: 30075815 Free PMC article. - The roles of resident, central and effector memory CD4 T-cells in protective immunity following infection or vaccination.
Gray JI, Westerhof LM, MacLeod MKL. Gray JI, et al. Immunology. 2018 Mar 23;154(4):574-81. doi: 10.1111/imm.12929. Online ahead of print. Immunology. 2018. PMID: 29570776 Free PMC article. Review. - Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease.
DuPage M, Bluestone JA. DuPage M, et al. Nat Rev Immunol. 2016 Mar;16(3):149-63. doi: 10.1038/nri.2015.18. Epub 2016 Feb 15. Nat Rev Immunol. 2016. PMID: 26875830 Review.
References
- Murphy K M, Ouyang W, Farrar J D, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy T L. Annu Rev Immunol. 2000;18:451–494. - PubMed
- Gray D. Nat Immunol. 2000;1:11–12. - PubMed
- Mosman T R, Coffman R L. Annu Rev Immunol. 1989;7:145–173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials