Proteomic analysis of the mammalian nuclear pore complex - PubMed (original) (raw)
Proteomic analysis of the mammalian nuclear pore complex
Janet M Cronshaw et al. J Cell Biol. 2002.
Abstract
As the sole site of nucleocytoplasmic transport, the nuclear pore complex (NPC) has a vital cellular role. Nonetheless, much remains to be learned about many fundamental aspects of NPC function. To further understand the structure and function of the mammalian NPC, we have completed a proteomic analysis to identify and classify all of its protein components. We used mass spectrometry to identify all proteins present in a biochemically purified NPC fraction. Based on previous characterization, sequence homology, and subcellular localization, 29 of these proteins were classified as nucleoporins, and a further 18 were classified as NPC-associated proteins. Among the 29 nucleoporins were six previously undiscovered nucleoporins and a novel family of WD repeat nucleoporins. One of these WD repeat nucleoporins is ALADIN, the gene mutated in triple-A (or Allgrove) syndrome. Our analysis defines the proteome of the mammalian NPC for the first time and paves the way for a more detailed characterization of NPC structure and function.
Figures
Figure 1.
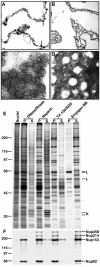
Fractionation of rat liver nuclei. (A–D) EM analysis of the fractionation of rat liver nuclei. Bar, 100 nm. (A) Thin-section EM of pelleted nuclear envelopes following digestion with DNase/RNase. Arrowheads indicate electron-dense aggregates associated with the inner nuclear membrane. (B) Thin-section EM of nuclear envelopes after extraction with heparin. (C) Negative staining EM of the pellet after extraction with Triton X-100/SDS. (D) Negative staining EM of the pellet after extraction with Empigen BB. (E) Silver stained SDS-PAGE analysis of supernatant (S) and pellet (P) from each step of the fractionation. Molecular weight markers are shown on the left. Histones (H) and lamins (L) are denoted on the right. (F) Immunoblot analysis of the pellet and supernatant from each step of the fractionation using mAb414 which recognizes the nucleoporins Nup358, Nup214, Nup153, and Nup62 (Davis and Blobel, 1986). Molecular weight markers are indicated on the left and nucleoporins are indicated on the right.
Figure 2.
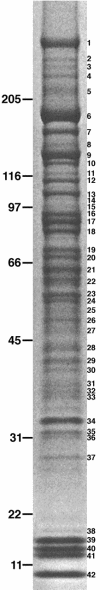
Identification of bands from unseparated Empigen BB supernatant. 10 U of Empigen BB supernatant were separated by SDS-PAGE and stained with Coomassie. Molecular weight markers are shown on the left and bands excised for mass spectrometric analysis are indicated on the right. Proteins identified in these bands are shown in Table I.
Figure 3.
Chromatographic separation of the Empigen BB supernatant. ∼1,000 U of C4-separated Empigen BB supernatant fractions were separated by SDS-PAGE and stained with Coomassie. Molecular weight markers are shown on the left and bands excised for mass spectrometric analysis are indicated to the right of each band. Proteins identified in these bands are shown in Tables I and SI, available at
http:www.jcb.org/cgi/content/full/jcb.200206106/DC1
(histones are not shown on this gel).
Figure 4.
Subcellular localization of uncharacterized proteins. GFP-tagged fusion proteins were transiently transfected into HeLa cells and their localization visualized by confocal microscopy 48 h posttransfection (green, left). Transfected cells were also labeled with mAb414 (red, middle). Merged images showing the extent of colocalization are shown in the right panel.
Similar articles
- Functional architecture of the nuclear pore complex.
Grossman E, Medalia O, Zwerger M. Grossman E, et al. Annu Rev Biophys. 2012;41:557-84. doi: 10.1146/annurev-biophys-050511-102328. Annu Rev Biophys. 2012. PMID: 22577827 Review. - Identification and characterization of nuclear pore complex components in Arabidopsis thaliana.
Tamura K, Fukao Y, Iwamoto M, Haraguchi T, Hara-Nishimura I. Tamura K, et al. Plant Cell. 2010 Dec;22(12):4084-97. doi: 10.1105/tpc.110.079947. Epub 2010 Dec 28. Plant Cell. 2010. PMID: 21189294 Free PMC article. - Architecture of the symmetric core of the nuclear pore.
Lin DH, Stuwe T, Schilbach S, Rundlet EJ, Perriches T, Mobbs G, Fan Y, Thierbach K, Huber FM, Collins LN, Davenport AM, Jeon YE, Hoelz A. Lin DH, et al. Science. 2016 Apr 15;352(6283):aaf1015. doi: 10.1126/science.aaf1015. Epub 2016 Apr 14. Science. 2016. PMID: 27081075 Free PMC article. - Molecular architecture of the inner ring scaffold of the human nuclear pore complex.
Kosinski J, Mosalaganti S, von Appen A, Teimer R, DiGuilio AL, Wan W, Bui KH, Hagen WJ, Briggs JA, Glavy JS, Hurt E, Beck M. Kosinski J, et al. Science. 2016 Apr 15;352(6283):363-5. doi: 10.1126/science.aaf0643. Science. 2016. PMID: 27081072 Free PMC article. - Structure and Assembly of the Nuclear Pore Complex.
Hampoelz B, Andres-Pons A, Kastritis P, Beck M. Hampoelz B, et al. Annu Rev Biophys. 2019 May 6;48:515-536. doi: 10.1146/annurev-biophys-052118-115308. Epub 2019 Apr 3. Annu Rev Biophys. 2019. PMID: 30943044 Review.
Cited by
- Atomic structure of the Y complex of the nuclear pore.
Kelley K, Knockenhauer KE, Kabachinski G, Schwartz TU. Kelley K, et al. Nat Struct Mol Biol. 2015 May;22(5):425-431. doi: 10.1038/nsmb.2998. Epub 2015 Mar 30. Nat Struct Mol Biol. 2015. PMID: 25822992 Free PMC article. - To the pore and through the pore: a story of mRNA export kinetics.
Oeffinger M, Zenklusen D. Oeffinger M, et al. Biochim Biophys Acta. 2012 Jun;1819(6):494-506. doi: 10.1016/j.bbagrm.2012.02.011. Epub 2012 Feb 22. Biochim Biophys Acta. 2012. PMID: 22387213 Free PMC article. Review. - The yeast nuclear pore complex and transport through it.
Aitchison JD, Rout MP. Aitchison JD, et al. Genetics. 2012 Mar;190(3):855-83. doi: 10.1534/genetics.111.127803. Genetics. 2012. PMID: 22419078 Free PMC article. - Long-term clinical follow-up and molecular genetic findings in eight patients with triple A syndrome.
Dumic M, Barišic N, Kusec V, Stingl K, Skegro M, Stanimirovic A, Koehler K, Huebner A. Dumic M, et al. Eur J Pediatr. 2012 Oct;171(10):1453-9. doi: 10.1007/s00431-012-1745-1. Epub 2012 Apr 28. Eur J Pediatr. 2012. PMID: 22538409 - Deficiency of ferritin heavy-chain nuclear import in triple a syndrome implies nuclear oxidative damage as the primary disease mechanism.
Storr HL, Kind B, Parfitt DA, Chapple JP, Lorenz M, Koehler K, Huebner A, Clark AJ. Storr HL, et al. Mol Endocrinol. 2009 Dec;23(12):2086-94. doi: 10.1210/me.2009-0056. Epub 2009 Oct 23. Mol Endocrinol. 2009. PMID: 19855093 Free PMC article.
References
- Allen, T.D., J.M. Cronshaw, S. Bagley, E. Kiseleva, and M.W. Goldberg. 2000. The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J. Cell Sci. 113:1651–1659. - PubMed
- Blobel, G., and V.R. Potter. 1966. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 154:1662–1665. - PubMed
- Cai, S.T., F.L. Zhou, and J.Z. Zhang. 1997. Immunogold labeling electron microscopy showing vimentin filament anchored on nuclear pore complex. Shi Yan Sheng Wu Xue Bao. 30:193–199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous