T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking - PubMed (original) (raw)
T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking
P Kivisäkk et al. Clin Exp Immunol. 2002 Sep.
Abstract
It is believed that chemokines and their receptors are involved in trafficking of T-cells to the central nervous system (CNS). The aim of the current study was to define the expression on cerebrospinal fluid (CSF) T-cells of six chemokine receptors associated with trafficking to sites of inflammation. Flow cytometry was used to detect chemokine receptor expression. We observed that CD3+T-cells in the CSF express a restricted array of inflammatory chemokine receptors, specifically CXCR3, CCR5 and CCR6, but little CCR1-3. This repertoire was independent of the presence of CNS inflammation, since comparable findings were obtained in patients with multiple sclerosis (MS) and individuals with non-inflammatory neurological diseases. The enrichment of CCR5+T-cells in the CSF could largely be explained by higher frequency of CD4+/CD45RO+T-cells in this compartment. In contrast, CD4+/CD45RO+T-cells expressing CXCR3 were significantly enriched in CSF as compared with blood. Similar levels of CCR6+/CD3+T-cells were observed in blood and CSF, while levels of CCR2+/CD3+T-cells were lower in CSF than in blood. The CSF was virtually devoid of CCR5+/CXCR3- T-cells, suggesting that the expression of CCR5 alone is not sufficient for the trafficking of CD3+T-cells to the CSF. We hypothesize that CXCR3 is the principal inflammatory chemokine receptor involved in intrathecal accumulation of T-cells in MS. Through interactions with its ligands, CXCR3 is proposed to mediate retention of T-cells in the inflamed CNS.
Figures
Fig. 1
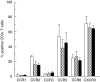
Chemokine receptor expression on CD3+T-cells was compared in stainings of whole blood, followed by selective erythrocyte lysis, performed at RT (□) and +4°C ( ), and in stainings of PBMCs performed at RT (▪). Figure shows mean +SD of three healthy donors.
), and in stainings of PBMCs performed at RT (▪). Figure shows mean +SD of three healthy donors.
Fig. 2
Cells from peripheral blood (PB) and CSF were gated according to forward- and side light scattering properties, and were positively selected for CD3 expression. Histograms for each chemokine receptor on CSF T-cells (——) are shown overlaid on the paired staining of PB T-cells (–––––) from the same donor. Isotype matched control moAbs were used for each chemokine receptor.
Fig. 3
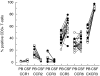
Chemokine receptor expression was determined on CD3+T-cells in peripheral blood (PB) and CSF from patients with inflammatory CNS demyelination (manifested as MS (21 patients), a clinically isolated syndrome suggestive of MS (10 patients), or recurrent multicentric myelitis (1 patient); (○) and control individuals with non-inflammatory neurological disorders (NIND; •) using flow cytometry. Lines connect paired blood and CSF samples from individual patients.
Fig. 4
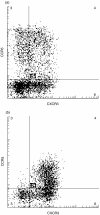
Co-expression of CXCR3 and CCR5 on individual T-cells in (a) peripheral blood and (b) CSF was compared using three-colour flow cytometry. Cells were gated according to forward- and side-light scattering properties, and were positively selected for CD3 expression.
Similar articles
- Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS.
Giunti D, Borsellino G, Benelli R, Marchese M, Capello E, Valle MT, Pedemonte E, Noonan D, Albini A, Bernardi G, Mancardi GL, Battistini L, Uccelli A. Giunti D, et al. J Leukoc Biol. 2003 May;73(5):584-90. doi: 10.1189/jlb.1202598. J Leukoc Biol. 2003. PMID: 12714572 - Selective lymphocyte chemokine receptor expression in the rheumatoid joint.
Ruth JH, Rottman JB, Katschke KJ Jr, Qin S, Wu L, LaRosa G, Ponath P, Pope RM, Koch AE. Ruth JH, et al. Arthritis Rheum. 2001 Dec;44(12):2750-60. doi: 10.1002/1529-0131(200112)44:12<2750::aid-art462>3.0.co;2-c. Arthritis Rheum. 2001. PMID: 11762935 - Abnormal expression of chemokine receptors in Behçet's disease: relationship to intracellular Th1/Th2 cytokines and to clinical manifestations.
Houman H, Hamzaoui A, Ben Ghorbal I, Khanfir M, Feki M, Hamzaoui K. Houman H, et al. J Autoimmun. 2004 Nov;23(3):267-73. doi: 10.1016/j.jaut.2004.07.005. J Autoimmun. 2004. PMID: 15501397 - [Chemokines and chemokine receptors in multiple sclerosis].
Misu T, Fujihara K, Itoyama Y. Misu T, et al. Nihon Rinsho. 2003 Aug;61(8):1422-7. Nihon Rinsho. 2003. PMID: 12962033 Review. Japanese. - Chemokine receptor CXCR3: an unexpected enigma.
Liu L, Callahan MK, Huang D, Ransohoff RM. Liu L, et al. Curr Top Dev Biol. 2005;68:149-81. doi: 10.1016/S0070-2153(05)68006-4. Curr Top Dev Biol. 2005. PMID: 16124999 Review.
Cited by
- Differences in systemic and central nervous system cellular immunity relevant to relapsing-remitting multiple sclerosis.
Matsui M, Araya S, Wang HY, Matsushima K, Saida T. Matsui M, et al. J Neurol. 2005 Aug;252(8):908-15. doi: 10.1007/s00415-005-0778-z. Epub 2005 Mar 21. J Neurol. 2005. PMID: 15772738 - Immune-cell crosstalk in multiple sclerosis.
Ransohoff RM. Ransohoff RM. Nature. 2018 Nov;563(7730):194-195. doi: 10.1038/d41586-018-07063-z. Nature. 2018. PMID: 30390068 No abstract available. - Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis.
Schafflick D, Xu CA, Hartlehnert M, Cole M, Schulte-Mecklenbeck A, Lautwein T, Wolbert J, Heming M, Meuth SG, Kuhlmann T, Gross CC, Wiendl H, Yosef N, Meyer Zu Horste G. Schafflick D, et al. Nat Commun. 2020 Jan 14;11(1):247. doi: 10.1038/s41467-019-14118-w. Nat Commun. 2020. PMID: 31937773 Free PMC article. - B and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers.
van Langelaar J, Rijvers L, Smolders J, van Luijn MM. van Langelaar J, et al. Front Immunol. 2020 May 8;11:760. doi: 10.3389/fimmu.2020.00760. eCollection 2020. Front Immunol. 2020. PMID: 32457742 Free PMC article. Review. - Differential effects of anti-CD20 therapy on CD4 and CD8 T cells and implication of CD20-expressing CD8 T cells in MS disease activity.
Shinoda K, Li R, Rezk A, Mexhitaj I, Patterson KR, Kakara M, Zuroff L, Bennett JL, von Büdingen HC, Carruthers R, Edwards KR, Fallis R, Giacomini PS, Greenberg BM, Hafler DA, Ionete C, Kaunzner UW, Lock CB, Longbrake EE, Pardo G, Piehl F, Weber MS, Ziemssen T, Jacobs D, Gelfand JM, Cross AH, Cameron B, Musch B, Winger RC, Jia X, Harp CT, Herman A, Bar-Or A. Shinoda K, et al. Proc Natl Acad Sci U S A. 2023 Jan 17;120(3):e2207291120. doi: 10.1073/pnas.2207291120. Epub 2023 Jan 12. Proc Natl Acad Sci U S A. 2023. PMID: 36634138 Free PMC article.
References
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–9. - PubMed
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–85. - PubMed
- Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain. 1999;122:2279–95. - PubMed
- Bitsch A, Wegener C, da Costa C, et al. Lesion development in Marburg's type of acute multiple sclerosis: from inflammation to demyelination. Mult Scler. 1999;5:138–46. - PubMed
- Luster AD. Chemokines – chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials