Timing of APC/C substrate degradation is determined by fzy/fzr specificity of destruction boxes - PubMed (original) (raw)
Comparative Study
Timing of APC/C substrate degradation is determined by fzy/fzr specificity of destruction boxes
Amit Zur et al. EMBO J. 2002.
Abstract
The anaphase promoting complex/cyclosome (APC/C), activated by fzy and fzr, degrades cell cycle proteins that carry RXXL or KEN destruction boxes (d-boxes). APC/C substrates regulate sequential events and must be degraded in the correct order during mitosis and G(1). We studied how d-boxes determine APC/C(fzy)/APC/C(fzr) specificity and degradation timing. Cyclin B1 has an RXXL box and is degraded by both APC/C(fzy) and APC/C(fzr); fzy has a KEN box and is degraded by APC/C(fzr) only. We characterized the degradation of substrates with swapped d-boxes. Cyclin B1 with KEN was degraded by APC/C(fzr) only. Fzy with RXXL could be degraded by APC/C(fzy) and APC/C(fzr). Interestingly, APC/C(fzy)- but not APC/C(fzr)-specific degradation is highly dependent on the location of RXXL. We studied degradation of tagged substrates in real time and observed that APC/C(fzr) is activated in early G(1). These observations demonstrate how d-box specificities of APC/C(fzy) and APC/C(fzr), and the successive activation of APC/C by fzy and fzr, establish the temporal degradation pattern. Our observations can explain further why some endogenous RXXL substrates are degraded by APC/C(fzy), while others are restricted to APC/C(fzr).
Figures
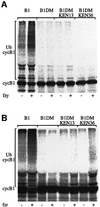
Fig. 1. A KEN box can target cyclin B1 for degradation in G1. Expression vectors for CAT, B1–CAT, its RXXL box (GTAV) mutant B1DM–CAT, and B1DM-KEN-CAT that has both a GTAV and an artificial KEN, were stably expressed in NIH 3T3 fibroblasts. Prometaphase-arrested cells, obtained by nocodazole treatment and subsequent shake-off, were released into fresh medium, harvested at the indicated time points and assayed for CAT activity (A). Cells stably expressing analogous constructs with a GFP reporter were synchronized in prometaphase and in G1. Degradation of wild-type and mutant B1–GFP reporters was analyzed by immunoblotting with GFP antibodies. Endogenous cyclin B1 was probed in parallel to establish that cells did indeed enter G1. Actin served as a loading control (B). KEN boxes were introduced at the indicated sites of the N-terminus of B1DM-CAT. The degradation of these reporters was assayed upon release into G1, but only KEN36, as used in (A), was degraded in G1 (C).
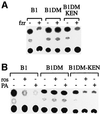
Fig. 2. Cyclin B1DM-KEN can be degraded in prometaphase by fzr overexpression and by cdk1 inhibition. Cells were transiently co-transfected with the indicated reporters together with an empty (–) or a human fzr (+) expression vector in a 1:3 ratio, respectively. After 24 h, cells were treated with nocodazole for 16 h. Prometaphase-arrested cells were obtained by shake-off and assayed for CAT activity (A). Cells stably expressing the indicated reporters were treated with nocodazole for 16 h, and prometaphase cells obtained by shake-off were transferred to fresh medium with nocodazole with or without roscovitin (ros) or purvalanol A (PA). Cells were harvested after 3 h and assayed for CAT activity (B).
Fig. 3. Cyclin B1 with a mutated RXXL and a KEN box is ubiquitylated in vitro by APC/Cfzr. CAT fusion proteins of cyclin B1, B1DM and B1DM with KEN boxes at positions 13 or 36 only were ubiquitylated in vitro with mitotic APC/C with (+) or without (–) fzy (A) or with interphase APC/C with (+) or without (–) fzr (B).
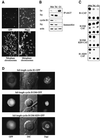
Fig. 4. Cyclin B1 with a mutated RXXL box and a KEN box is degraded in vivo by APC/Cfzr only. Cells were transfected with an expression vector for non-degradable catalytically active cyclin B1–GFP to arrest them in telophase. Almost all the round mitotic cells observed by phase–contrast microscopy are GFP positive (top panels). More than 90% of chromosome spreads prepared from cells arrested by this transfection had fully separated sister chromatids (bottom left panel), compared with only a small fraction that had non-separated metaphase chromosomes (bottom right panel). These results indicate that this protocol yields highly synchronous populations of telophase-arrested cells (A). Cells arrested in telophase by this protocol, and in prometaphase with nocodazole were obtained by gentle shake-off. G1 cells were obtained by growing prometaphase cells obtained by shake-off for 4 h in fresh medium. The APC/C complex was immunoprecipitated with cdc27 antibodies from extracts of prometaphase, telophase and G1 cells and probed with fzy and fzr antibodies. Cell extracts were also directly probed with the indicated antibodies (B). Cells stably expressing the indicated reporters were synchronized as described in (B) and assayed for CAT activity (C). A KEN36 box was introduced into the full-length cyclin B1DM–GFP used in (A). Cells were transfected with this vector and with wild-type and non-degradable cyclin B1–GFP expression vectors on a glass-bottom culture dish. Living transfected cells were photographed after 40 h with fluorescence (GFP and DAPI) and DIC illumination at 630× magnification (D).
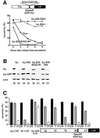
Fig. 5. The RXXL box can replace the KEN box of fzy and change its fzy/fzr specificity. Degradation of fzy-CAT (fzy) and a mutant with alanine replacement of the KEN box (fzy-KM) was analyzed in cells stably expressing vectors for these fusion reporters. Degradation of fzy-KM either with a cyclin B1 RXXL box (fzy-KM-RXXL99), or with a mutated RXXL box at the same location (fzy-KM-DM) was tested in the same way (A). Degradation of fzy–GFP and its derivatives upon release into G1 was assayed by immunoblotting of cells stably expressing fusion reporters. Mitotic cells were obtained by nocodazole arrest and shake-off, and G1 cells by release of mitotic cells into G1 for 4 h. Endogenous fzy and actin were probed in parallel (B). RXXL boxes were introduced into the indicated locations of the non-degradable fzy-KM-CAT reporter. The CAT activity of these reporters was assayed in prometaphase (M), telophase (T) and G1. This experiment was performed in triplicate (see error bars). All CAT fusion proteins were stable in prometaphase and degraded in G1 by APC/Cfzr. In contrast, only the fzy-KM with an RXXL box introduced into position 78 was degraded as efficiently as cyclin B1 by the APC/Cfzy in telophase. Fzy-KM with RXXL in position 99 was hardly degraded by the APC/Cfzy (C), like wild-type fzy-CAT.
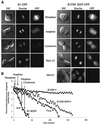
Fig. 6. Live cell imaging of the initiation of APC/Cfzy and APC/Cfzr activity. Cells stably expressing B1–GFP and B1DM-KEN–GFP were photographed at the indicated stages of mitosis and G1 at 630× magnification with GFP and DAPI filter sets, as well as by DIC optics. The mid-G1 cells were followed through mitosis and photographed 3 h after metaphase (A). Cells stably expressing GFP fusion proteins of cyclin B1, B1DM, B1-KEN, B1DM-KEN and fzy were followed through mitosis and photographed every 3 min. Background-subtracted GFP fluorescence was analyzed as described in Materials and methods and plotted as a function of time (B). The stages of mitosis are indicated by arrows.
Similar articles
- The roles of Fzy/Cdc20 and Fzr/Cdh1 in regulating the destruction of cyclin B in space and time.
Raff JW, Jeffers K, Huang JY. Raff JW, et al. J Cell Biol. 2002 Jun 24;157(7):1139-49. doi: 10.1083/jcb.200203035. Epub 2002 Jun 24. J Cell Biol. 2002. PMID: 12082076 Free PMC article. - Mammalian Cdh1/Fzr mediates its own degradation.
Listovsky T, Oren YS, Yudkovsky Y, Mahbubani HM, Weiss AM, Lebendiker M, Brandeis M. Listovsky T, et al. EMBO J. 2004 Apr 7;23(7):1619-26. doi: 10.1038/sj.emboj.7600149. Epub 2004 Mar 18. EMBO J. 2004. PMID: 15029244 Free PMC article. - The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1.
Pfleger CM, Kirschner MW. Pfleger CM, et al. Genes Dev. 2000 Mar 15;14(6):655-65. Genes Dev. 2000. PMID: 10733526 Free PMC article. - Mechanisms and regulation of the degradation of cyclin B.
Hershko A. Hershko A. Philos Trans R Soc Lond B Biol Sci. 1999 Sep 29;354(1389):1571-5; discussion 1575-6. doi: 10.1098/rstb.1999.0500. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10582242 Free PMC article. Review. - To cell cycle, swing the APC/C.
van Leuken R, Clijsters L, Wolthuis R. van Leuken R, et al. Biochim Biophys Acta. 2008 Sep;1786(1):49-59. doi: 10.1016/j.bbcan.2008.05.002. Epub 2008 May 21. Biochim Biophys Acta. 2008. PMID: 18544349 Review.
Cited by
- APC(FZR1) prevents nondisjunction in mouse oocytes by controlling meiotic spindle assembly timing.
Holt JE, Lane SI, Jennings P, García-Higuera I, Moreno S, Jones KT. Holt JE, et al. Mol Biol Cell. 2012 Oct;23(20):3970-81. doi: 10.1091/mbc.E12-05-0352. Epub 2012 Aug 23. Mol Biol Cell. 2012. PMID: 22918942 Free PMC article. - Uncoupling anaphase-promoting complex/cyclosome activity from spindle assembly checkpoint control by deregulating polo-like kinase 1.
van de Weerdt BC, van Vugt MA, Lindon C, Kauw JJ, Rozendaal MJ, Klompmaker R, Wolthuis RM, Medema RH. van de Weerdt BC, et al. Mol Cell Biol. 2005 Mar;25(5):2031-44. doi: 10.1128/MCB.25.5.2031-2044.2005. Mol Cell Biol. 2005. PMID: 15713655 Free PMC article. - APOLLON protein promotes early mitotic CYCLIN A degradation independent of the spindle assembly checkpoint.
Kikuchi R, Ohata H, Ohoka N, Kawabata A, Naito M. Kikuchi R, et al. J Biol Chem. 2014 Feb 7;289(6):3457-67. doi: 10.1074/jbc.M113.514430. Epub 2013 Dec 3. J Biol Chem. 2014. PMID: 24302728 Free PMC article. - A novel RING finger protein, human enhancer of invasion 10, alters mitotic progression through regulation of cyclin B levels.
Toby GG, Gherraby W, Coleman TR, Golemis EA. Toby GG, et al. Mol Cell Biol. 2003 Mar;23(6):2109-22. doi: 10.1128/MCB.23.6.2109-2122.2003. Mol Cell Biol. 2003. PMID: 12612082 Free PMC article. - A novel motif governs APC-dependent degradation of Drosophila ORC1 in vivo.
Araki M, Yu H, Asano M. Araki M, et al. Genes Dev. 2005 Oct 15;19(20):2458-65. doi: 10.1101/gad.1361905. Epub 2005 Sep 29. Genes Dev. 2005. PMID: 16195415 Free PMC article.
References
- Ausubel F.M., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (1994) Current Protocols in Molecular Biology. John Wiley & Sons, New York, NY.
- Baumer M., Braus,G.H. and Irniger,S. (2000) Two different modes of cyclin clb2 proteolysis during mitosis in Saccharomyces cerevisiae. FEBS Lett., 468, 142–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous