X-linked thrombocytopenia with thalassemia from a mutation in the amino finger of GATA-1 affecting DNA binding rather than FOG-1 interaction - PubMed (original) (raw)
X-linked thrombocytopenia with thalassemia from a mutation in the amino finger of GATA-1 affecting DNA binding rather than FOG-1 interaction
Channing Yu et al. Blood. 2002.
Abstract
Transcription factor GATA-1 is essential for the development of erythroid cells and megakaryocytes. Each of its 2 zinc fingers is critical for normal function. The C-terminal finger is necessary for DNA binding. The N finger mediates interaction with FOG-1, a cofactor for GATA-1, and also modulates DNA-binding affinity, notably at complex or palindromic GATA sites. Residues of the N finger-mediating interaction with FOG-1 lie on the surface of the N finger facing away from DNA. Strong sequence conservation of residues facing DNA suggests that this other surface may also have an important role. We report here that a syndrome of X-linked thrombocytopenia with thalassemia in humans is caused by a missense mutation (Arg216Gln) in the GATA-1 N finger. To investigate the functional consequences of this substitution, we used site-directed mutagenesis to alter the corresponding residue in GATA-1. Compared with wild-type GATA-1, Arg216Gln GATA-1 shows comparable affinity to single GATA sites but decreased affinity to palindromic sites. Arg216Gln GATA-1 interacts with FOG-1 similarly with wild-type GATA-1. Arg216Gln GATA-1 supports erythroid maturation of GATA-1 erythroid cells, albeit at reduced efficiency compared with wild-type GATA-1. Together, these findings suggest that residues of the N finger of GATA-1-facing DNA contribute to GATA-1 function apart from interaction with the cofactor FOG-1. This is also the first example of beta-thalassemia in humans caused by a mutation in an erythroid transcription factor.
Figures
Figure 1. X-linked thrombocytopenia with thalassemia maps to a mutation (Arg216Gln) in GATA-1
(A) Pedigree of a family with X-linked thrombocytopenia with thalassemia., Black squares represent affected males; circles with black discs represent obligate carrier females. Differences in GATA-1 DNA sequence at codon 216 are indicated below individuals: CGG (arginine, wild-type); CAG (glutamine, mutant). Patients who were evaluated for α-globin/β-globin chain imbalance, are designated with + (elevated α/β ratio) or − (within normal limits). (B) Partial sequence of the N finger of GATA-1. Arg216 is conserved across GATA-1 orthologs of various species. Here, amino acid numbering relates to the human, rat, and mouse polypeptides. Val205, mutated to methionine in familial dyserythropoietic anemia with thrombocytopenia, is also highlighted here. Polypeptide sequences are shown as single-letter code. (C) The N finger of GATA-1 has 2 faces, a FOG face and a DNA face., Approximate division of these faces is superimposed on the NMR structure. Val205 (green) is highlighted on the FOG face, and Arg216 (blue) is highlighted on the DNA face.
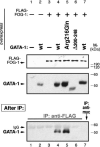
Figure 2. Arg216Gln GATA-1, like wild-type GATA-1, binds to FOG-1
A FLAG-tagged FOG-1 expression construct was cotransfected with various GATA-1 expression constructs into COS cells as indicated. Western blots (top panels) confirm expression from FOG and GATA constructs. (bottom panel) After immunoprecipitation (IP) with anti-FLAG antibody (or anti-myc control antibody), Western blot detection of precipitated GATA-1 shows that Arg216Gln GATA-1 (lane 5), like wild-type GATA-1, binds to FOG-1 (lane 4), whereas Δ200 to 248 GATA-1 (N finger–deleted; lane 6) do not. No immunoprecipitation of GATA-1 is observed in control experiments (remaining lanes).
Figure 3. Arg216Gln GATA-1 dissociates from a palindromic GATA site more easily than wild-type GATA-1
GATA-1∷DNA complexes were formed by incubating either wild-type or Arg216Gln GATA-1 with 32P-labeled single GATA site or palindromic GATA site probes (top). One hundred–fold molar excess of corresponding unlabeled probe was then added at t = 0 minute, and reaction mixtures were loaded onto gels at the indicated times (middle panels). The first lane of each set shows migration of the free probe alone. (bottom panels) PhosphorImager quantitation of percentage of bound probe over time (here, in seconds).
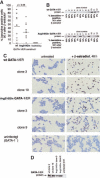
Figure 4. Arg216Gln GATA-1 allows erythroid maturation in G1E cells
G1E cells, which lack GATA-1, undergo erythroid differentiation after retroviral infection with GATA-1. Stable introduction of estrogen-receptor fusion versions of GATA-1 (GATA-1/ER) allows this differentiation to be controlled by the addition of β-estradiol, which activates the GATA-1/ER fusion protein., (A) Wild-type and Arg216Gln versions of GATA-1/ER fusion proteins allow G1E cells to undergo erythroid differentiation, evidenced here by quantitation of benzidine-positive cells 90 hours after induction by β-estradiol of 12 independent clones from each group. The mean percentage of benzidine-positive cells is slightly lower in the Arg216Gln clones (t test, P = .06). No benzidine-positive cells are observed in the Val205Gly clones. (B) GATA-1/ER protein is expressed at varying levels in wild-type (top) and Arg216 (bottom) clones. Corresponding percentages of benzidine-positive cells 90 hours after induction by β-estradiol (from panel A) in individual clones are indicated below the blots. (C) Benzidine staining of clones of G1E cells following stable introduction of wild-type GATA-1/ER (the ensuing clones are named G1E-ER2) or Arg216Gln GATA-1/ER, or G1E cells alone. Benzidine-positive cells (black) are visible after β-estradiol treatment (right panels) when wt GATA-1/ER or Arg216Gln GATA-1/ER is present. Origninal magnification, × 200. (D) GATA-1/ER protein levels of clones pictured in panel C.
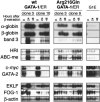
Figure 5. Changes in gene expression following activation of wild-type or Arg216Gln G1E-ER2 by β-estradiol
Two clones of wild-type or Arg216Gln G1E-ER2 cells were induced with β-estradiol, and transcripts of erythroid genes were assayed by Northern blotting. Like wild-type G1E-ER2 clones, Arg216Gln G1E-ER2 clones showed changes in erythroid gene expression. However, in some instances (eg, the erythroid anion transporter band 3; see “Results” for details), the level of induction was reduced relative to wild-type controls. Parental G1E cells, which lack GATA-1, do not display changes in expression of these markers following β-estradiol induction.
Similar articles
- X-linked thrombocytopenia caused by a novel mutation of GATA-1.
Mehaffey MG, Newton AL, Gandhi MJ, Crossley M, Drachman JG. Mehaffey MG, et al. Blood. 2001 Nov 1;98(9):2681-8. doi: 10.1182/blood.v98.9.2681. Blood. 2001. PMID: 11675338 - Transgenic rescue of GATA-1-deficient mice with GATA-1 lacking a FOG-1 association site phenocopies patients with X-linked thrombocytopenia.
Shimizu R, Ohneda K, Engel JD, Trainor CD, Yamamoto M. Shimizu R, et al. Blood. 2004 Apr 1;103(7):2560-7. doi: 10.1182/blood-2003-07-2514. Epub 2003 Dec 4. Blood. 2004. PMID: 14656885 - Dyserythropoietic anemia and thrombocytopenia due to a novel mutation in GATA-1.
Del Vecchio GC, Giordani L, De Santis A, De Mattia D. Del Vecchio GC, et al. Acta Haematol. 2005;114(2):113-6. doi: 10.1159/000086586. Acta Haematol. 2005. PMID: 16103636 - Coregulation of GATA factors by the Friend of GATA (FOG) family of multitype zinc finger proteins.
Cantor AB, Orkin SH. Cantor AB, et al. Semin Cell Dev Biol. 2005 Feb;16(1):117-28. doi: 10.1016/j.semcdb.2004.10.006. Epub 2004 Dec 15. Semin Cell Dev Biol. 2005. PMID: 15659346 Review. - Transcriptional regulation of erythropoiesis: an affair involving multiple partners.
Cantor AB, Orkin SH. Cantor AB, et al. Oncogene. 2002 May 13;21(21):3368-76. doi: 10.1038/sj.onc.1205326. Oncogene. 2002. PMID: 12032775 Review.
Cited by
- Analysis of disease-causing GATA1 mutations in murine gene complementation systems.
Campbell AE, Wilkinson-White L, Mackay JP, Matthews JM, Blobel GA. Campbell AE, et al. Blood. 2013 Jun 27;121(26):5218-27. doi: 10.1182/blood-2013-03-488080. Epub 2013 May 23. Blood. 2013. PMID: 23704091 Free PMC article. - GATA1 mutations in red cell disorders.
Ling T, Crispino JD. Ling T, et al. IUBMB Life. 2020 Jan;72(1):106-118. doi: 10.1002/iub.2177. Epub 2019 Oct 25. IUBMB Life. 2020. PMID: 31652397 Free PMC article. Review. - Human leukemia mutations corrupt but do not abrogate GATA-2 function.
Katsumura KR, Mehta C, Hewitt KJ, Soukup AA, Fraga de Andrade I, Ranheim EA, Johnson KD, Bresnick EH. Katsumura KR, et al. Proc Natl Acad Sci U S A. 2018 Oct 23;115(43):E10109-E10118. doi: 10.1073/pnas.1813015115. Epub 2018 Oct 9. Proc Natl Acad Sci U S A. 2018. PMID: 30301799 Free PMC article. - Combinatorial interactions of serpent, lozenge, and U-shaped regulate crystal cell lineage commitment during Drosophila hematopoiesis.
Fossett N, Hyman K, Gajewski K, Orkin SH, Schulz RA. Fossett N, et al. Proc Natl Acad Sci U S A. 2003 Sep 30;100(20):11451-6. doi: 10.1073/pnas.1635050100. Epub 2003 Sep 22. Proc Natl Acad Sci U S A. 2003. PMID: 14504400 Free PMC article. - Molecular Basis and Genetic Modifiers of Thalassemia.
Tesio N, Bauer DE. Tesio N, et al. Hematol Oncol Clin North Am. 2023 Apr;37(2):273-299. doi: 10.1016/j.hoc.2022.12.001. Hematol Oncol Clin North Am. 2023. PMID: 36907603 Free PMC article. Review.
References
- Orkin SH. Embryonic stem cells and transgenic mice in the study of hematopoiesis. Int J Dev Biol. 1998;42:927–934. - PubMed
- Vyas P, Ault K, Jackson CW, Orkin SH, Shivdasani RA. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood. 1999;93:2867–2875. - PubMed
- Lowry JA, Atchley WR. Molecular evolution of the GATA family of transcription factors: conservation within the DNA-binding domain. J Mol Evolution. 2000;50:103–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases