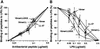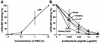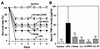Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues - PubMed (original) (raw)
Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues
Isao Nagaoka et al. Clin Diagn Lab Immunol. 2002 Sep.
Abstract
Mammalian myeloid and epithelial cells express various peptide antibiotics (such as defensins and cathelicidins) that contribute to the innate host defense against invading microorganisms. Among these peptides, human cathelicidin CAP18/LL-37 (L(1) to S(37)) possesses not only potent antibacterial activity against gram-positive and gram-negative bacteria but also the ability to bind to gram-negative lipopolysaccharide (LPS) and neutralize its biological activities. In this study, to develop peptide derivatives with improved LPS-neutralizing activities, we utilized an 18-mer peptide (K(15) to V(32)) of LL-37 as a template and evaluated the activities of modified peptides by using the CD14(+) murine macrophage cell line RAW 264.7 and the murine endotoxin shock model. By replacement of E(16) and K(25) with two L residues, the hydrophobicity of the peptide (18-mer LL) was increased, and by further replacement of Q(22), D(26), and N(30) with three K residues, the cationicity of the peptide (18-mer LLKKK) was enhanced. Among peptide derivatives, 18-mer LLKKK displayed the most powerful LPS-neutralizing activity: it was most potent at binding to LPS, inhibiting the interaction between LPS and LPS-binding protein, and attaching to the CD14 molecule, thereby suppressing the binding of LPS to CD14(+) cells and attenuating production of tumor necrosis factor alpha (TNF-alpha) by these cells. Furthermore, in the murine endotoxin shock model, 18-mer LLKKK most effectively suppressed LPS-induced TNF-alpha production and protected mice from lethal endotoxin shock. Together, these observations indicate that the LPS-neutralizing activities of the amphipathic human CAP18/LL-37-derived 18-mer peptide can be augmented by modifying its hydrophobicity and cationicity, and that 18-mer LLKKK is the most potent of the peptide derivatives, with therapeutic potential for gram-negative bacterial endotoxin shock.
Figures
FIG. 1.
Helical wheel projections for LL-37 and its 18-mer peptide derivatives. The sequences of α-helical peptides LL-37, 18-mer K15-V32, 18-mer LL, and 18-mer LLKKK are presented according to the Shiffer-Edmundson wheel projection analyzed with a Genetyx-Mac computer system (Software Development, Tokyo, Japan). To increase hydrophobicity, E16 and K25 in 18-mer K15-V32 were replaced by L16 and L25 in 18-mer LL, respectively. Furthermore, to increase cationicity, Q22, D26, and N30 in 18-mer LL were replaced by K22, K26, and K30 in 18-mer LLKKK, respectively. Positively charged residues are circled, whereas negatively charged residues are boxed. Hydrophobic residues are outlined, while neutral hydrophilic residues are not. The hydrophilic and hydrophobic sectors are divided by dashed lines.
FIG. 2.
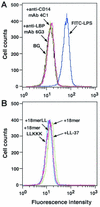
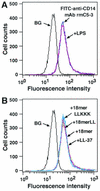
Effects of 18-mer peptides on binding of FITC-conjugated LPS to RAW 264.7 cells. (A) RAW 264.7 cells (5 × 105/ml) were incubated with 100 ng of FITC-conjugated LPS/ml in the absence or presence of 5 μg of anti-LBP MAb 6G3 or anti-CD14 MAb 4C1/ml in RPMI 1640 containing 10% FBS for 15 min at 37°C. (B) Alternatively, RAW 264.7 cells were incubated with FITC-conjugated LPS in the presence of 1 μg of peptide (18-mer K15-V32, 18-mer LL, 18-mer LLKKK, or LL-37)/ml in RPMI 1640 containing 10% FBS. After a wash, the binding of FITC-LPS was analyzed by flow cytometry. Background (BG) was assessed by using RAW 264.7 cells incubated without FITC-LPS. Data are from one of five separate experiments.
FIG. 3.
Dose-dependent inhibition of the binding of FITC-conjugated LPS to RAW 264.7 cells by 18-mer peptides. RAW 264.7 cells (5 × 105/ml) were incubated with 100 ng of FITC-conjugated LPS/ml in the absence or presence of 0.01 to 10 μg of 18-mer K15-V32, 18-mer LL, 18-mer LLKKK, or LL-37/ml in RPMI 1640 containing 10% FBS for 15 min at 37°C. After a wash, the binding of FITC-LPS was analyzed by flow cytometry, and median fluorescence intensity was determined. Binding of LPS was expressed as a percentage of that obtained by using RAW 264.7 cells incubated with FITC-conjugated LPS alone. Data are means ± SD from three to five separate experiments. Values for 18-mer K15-V32 are compared with those for 18-mer LL, 18-mer LLKKK, or LL-37. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
FIG. 4.
Effects of 18-mer peptides on LPS-induced TNF-α expression by RAW 264.7 cells. RAW 264.7 cells (106/well in a 24-well microplate) were incubated without (Control) or with 100 ng of LPS/ml in the absence (LPS) or presence of 1 μg of peptide (18-mer K15-V32, 18-mer LL, 18-mer LLKKK, or LL-37)/ml in 500 μl of RPMI 1640 containing 10% FBS for 4 h at 37°C. After incubation, cells were recovered, and expression of TNF-α mRNA and protein was analyzed by Northern (A) and Western (B) blotting, respectively. As a control, expression of β-actin mRNA and protein was also analyzed. Data are from one of three separate experiments.
FIG. 5.
Evaluation of LPS-binding activities of 18-mer peptides. (A) LPS-binding activities of the peptides were investigated by incubating 0.02 to 0.5 μg of 18-mer K15-V32, 18-mer LL, 18-mer LLKKK, or LL-37 in LPS-coated 96-well microtiter plates (100 ng of LPS/well) for 1 h at 37°C in 50 μl of RPMI 1640. Bound peptides were detected by TMB reaction by using a rabbit anti-CAP18 Ab and HRP-conjugated goat anti-rabbit IgG. (B) The peptide (0.1 μg of either 18-mer K15-V32, 18-mer LL, 18-mer LLKKK, or LL-37) was incubated in LPS-coated 96-well microtiter plates for 1 h at 37°C in the absence or presence of added LPS (0.05 to 2.5 μg/well) in 50 μl of RPMI 1640, and bound peptides were detected as described above. Binding of peptides to LPS-coated plates was expressed as a percentage of that observed when 0.5 μg (for panel A) or 0.1 μg (for panel B) of each peptide was incubated in the absence of added LPS. Data are means ± SD from three to five separate experiments. Values for 18-mer K15-V32 are compared with those for 18-mer LL, 18-mer LLKKK, or LL-37. ∗, P < 0.05; ∗∗∗, P < 0.001.
FIG. 6.
Effects of 18-mer peptides on the interaction of LPS with LBP. (A) LPS-LBP binding was examined by incubating 50 μl of RPMI 1640 containing 0.1, 1, or 10% FBS in LPS-coated 96-well microtiter plates (100 ng of LPS/well) for 1 h at 37°C. After incubation, bound LBP was detected by TMB reaction using anti-LBP MAb 6G3 and HRP-conjugated rabbit anti-mouse IgG. (B) LPS-coated microtiter plates were preincubated with either 18-mer K15-V32, 18-mer LL, 18-mer LLKKK, or LL-37 at 0.025 to 0.2 μg/well in 50 μl of RPMI 1640 for 1 h at 37°C. Thereafter, LPS-LBP binding was determined by incubating 50 μl of RPMI 1640 containing 10% FBS in the microtiter plates as described above. LPS-LBP binding was expressed as a percentage of that obtained by incubation with RPMI 1640 containing 10% FBS in the absence of added peptide. Data are means ± SD from three to eight separate experiments. Values for 18-mer K15-V32 are compared with those for 18-mer LL, 18-mer LLKKK, or LL-37. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
FIG. 7.
Effects of 18-mer peptides on expression of CD14 by RAW 264.7 cells. RAW 264.7 cells (5 × 105/ml) were incubated without or with 100 ng of LPS/ml (A) or 1 μg of peptide (18-mer K15-V32, 18-mer LL, 18-mer LLKKK, or LL-37)/ml (B) in RPMI 1640 containing 10% FBS for 15 min at 37°C, after which cells were further incubated with 2.5 μg of FITC-conjugated rat anti-mouse CD14 MAb rmC5-3/ml for 15 min at 37°C. After a wash, the binding of FITC-anti-CD14 MAb rmC5-3 was measured by flow cytometry. Background (BG) was assessed by using RAW 264.7 cells incubated with FITC-conjugated rabbit anti-mouse IgG (a negative control for nonspecific binding). Data are from one of three separate experiments.
FIG. 8.
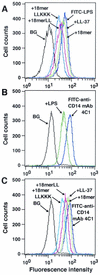
Evaluation of the binding of 18-mer peptides to CD14+ cells. (A) RAW 264.7 cells (5 × 105/ml) were preincubated with 1 μg of peptide (18-mer K15-V32, 18-mer LL, 18-mer LLKKK, or LL-37)/ml in RPMI 1640 containing 10% FBS for 10 min at 37°C. After a wash, cells were incubated with 100 ng of FITC-conjugated LPS/ml in RPMI 1640 containing 10% FBS for 15 min at 37°C, and the binding of FITC-conjugated LPS was analyzed by flow cytometry. Background (BG) was assessed by using RAW 264.7 cells incubated without FITC-conjugated LPS. (B and C) RAW 264.7 cells (5 × 105/ml) were preincubated without or with 100 ng of LPS/ml or 1 μg of peptide (18-mer K15-V32, 18-mer LL, 18-mer LLKKK, or LL-37)/ml for 15 min at 37°C in RPMI 1640 containing 10% FBS. Cells were further incubated with 50 ng of FITC-conjugated neutralizing anti-CD14 MAb 4C1/ml for 15 min at 37°C, and after a wash, the binding of FITC-conjugated anti-CD14 MAb 4C1 was analyzed by flow cytometry. Background (BG) was assessed by using RAW 264.7 cells incubated with FITC-conjugated rabbit anti-mouse IgG (a negative control for nonspecific binding). Data are from one of three to six separate experiments.
FIG. 9.
Protective effects of 18-mer peptides on survival and serum TNF-α levels in LPS-challenged mice. (A) Mice were i.p. injected with
d
-galactosamine alone at 18 mg/mouse (Control) or with LPS at 200 ng/mouse with or without 1 μg of peptide (18-mer K15-V32, 18-mer LL, 18-mer LLKKK, or LL-37)/mouse. After injection, deaths were recorded every 24 h until day 6 (16 mice in each group). (B) After LPS challenge (75 min), serum samples were prepared, and serum TNF-α levels were determined by using a commercially available mouse TNF-α enzyme-linked immunosorbent assay kit. Data are means ± SD for 16 mice in each group. Values without and with peptide administration are compared, as are values with and without LPS administration. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
FIG. 10.
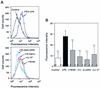
Effects of 18-mer peptide administration on the binding of FITC-conjugated LPS to mouse peritoneal macrophages. Mice were i.p. injected with
d
-galactosamine alone at 18 mg/mouse (Control) or with FITC-conjugated LPS at 200 ng/mouse with or without 1 μg of peptide (18-mer K15-V32, 18-mer LL, 18-mer LLKKK, or LL-37)/mouse. After LPS challenge (75 min), peritoneal macrophages were recovered, and binding of FITC-conjugated LPS to the cells was analyzed by flow cytometry. (A) Data are from 1 of 16 mice in each group. (B) Data are means ± SD for 16 mice in each group. Values without and with peptide administration are compared, as are values with and without LPS administration. ∗∗∗, P < 0.001.
FIG. 11.
Hydrophilicity/hydrophobicity plots of LL-37 and its 18-mer peptide derivatives. Hydropathy indices (+, hydrophilicity; −, hydrophobicity) of LL-37 and its derivatives (18-mer K15 to V32, 18-mer LL, and 18-mer LLKKK) were calculated by the algorithm of Hopp and Woods by use of a Genetyx-Mac computer system, and the pIs of the peptides were also determined by the same system. Horizontal axes display the amino acid position number.
Similar articles
- Augmentation of the bactericidal activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by amino acid substitutions.
Nagaoka I, Kuwahara-Arai K, Tamura H, Hiramatsu K, Hirata M. Nagaoka I, et al. Inflamm Res. 2005 Feb;54(2):66-73. doi: 10.1007/s00011-004-1323-8. Inflamm Res. 2005. PMID: 15750713 - Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14(+) cells.
Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, Heumann D. Nagaoka I, et al. J Immunol. 2001 Sep 15;167(6):3329-38. doi: 10.4049/jimmunol.167.6.3329. J Immunol. 2001. PMID: 11544322 - Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37.
Ciornei CD, Sigurdardóttir T, Schmidtchen A, Bodelsson M. Ciornei CD, et al. Antimicrob Agents Chemother. 2005 Jul;49(7):2845-50. doi: 10.1128/AAC.49.7.2845-2850.2005. Antimicrob Agents Chemother. 2005. PMID: 15980359 Free PMC article. - Cathelicidin LL-37: LPS-neutralizing, pleiotropic peptide.
Golec M. Golec M. Ann Agric Environ Med. 2007;14(1):1-4. Ann Agric Environ Med. 2007. PMID: 17655171 Review. - High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments.
Wang G, Mishra B, Epand RF, Epand RM. Wang G, et al. Biochim Biophys Acta. 2014 Sep;1838(9):2160-72. doi: 10.1016/j.bbamem.2014.01.016. Epub 2014 Jan 23. Biochim Biophys Acta. 2014. PMID: 24463069 Free PMC article. Review.
Cited by
- Intestinal biofilms: pathophysiological relevance, host defense, and therapeutic opportunities.
Jandl B, Dighe S, Gasche C, Makristathis A, Muttenthaler M. Jandl B, et al. Clin Microbiol Rev. 2024 Sep 12;37(3):e0013323. doi: 10.1128/cmr.00133-23. Epub 2024 Jul 12. Clin Microbiol Rev. 2024. PMID: 38995034 Review. - Regeneration of critical-sized defects, in a goat model, using a dextrin-based hydrogel associated with granular synthetic bone substitute.
Pereira I, Pereira JE, Maltez L, Rodrigues A, Rodrigues C, Oliveira M, Silva DM, Caseiro AR, Prada J, Maurício AC, Santos JD, Gama M. Pereira I, et al. Regen Biomater. 2020 Nov 28;8(1):rbaa036. doi: 10.1093/rb/rbaa036. eCollection 2021 Feb 1. Regen Biomater. 2020. PMID: 33732486 Free PMC article. - The Human LL-37(17-29) antimicrobial peptide reveals a functional supramolecular structure.
Engelberg Y, Landau M. Engelberg Y, et al. Nat Commun. 2020 Aug 4;11(1):3894. doi: 10.1038/s41467-020-17736-x. Nat Commun. 2020. PMID: 32753597 Free PMC article. - Determination of the antibacterial and lipopolysaccharide-neutralizing regions of guinea pig neutrophil cathelicidin peptide CAP11.
Okuda D, Yomogida S, Tamura H, Nagaoka I. Okuda D, et al. Antimicrob Agents Chemother. 2006 Aug;50(8):2602-7. doi: 10.1128/AAC.00331-06. Antimicrob Agents Chemother. 2006. PMID: 16870748 Free PMC article.
References
- Adachi, Y., C. Satokawa, M. Saeki, N. Ohno, H. Tamura, S. Tanaka, and T. Yadomae. 1999. Inhibition by a CD14 monoclonal antibody of lipopolysaccharide binding to murine macrophages. J. Endotoxin Res. 5:139-146.
- Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. - PubMed
- Beutler, B., I. W. Milsark, and A. C. Cerami. 1985. Passive immunization against cathectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 229:869-871. - PubMed
- Dankesreiter, S., A. Hoess, J. Schneider-Mergener, H. Wagner, and T. Miethke. 2000. Synthetic endotoxin-binding peptides block endotoxin-triggered TNF-α production by macrophages in vitro and in vivo and prevent endotoxin-mediated toxic shock. J. Immunol. 164:4804-4811. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous