Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation - PubMed (original) (raw)
Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation
Claudio Talora et al. Genes Dev. 2002.
Abstract
The Notch family of cell surface receptors plays a key role in cell-fate determination and differentiation, functioning in a cell- and context-specific manner. In mammalian cells, Notch activation is generally thought to maintain stem cell potential and inhibit differentiation, thereby promoting carcinogenesis. However, in other contexts such as primary epithelial cells (keratinocytes), increased Notch activity causes exit from the cell cycle and/or commitment to differentiation. We now report that expression of the endogenous Notch1 gene is markedly reduced in a panel of cervical carcinoma cells whereas expression of Notch2 remains elevated, and Notch1 expression is similarly reduced or absent in invasive cervical cancers. Conversely, expression of activated Notch1 causes strong growth inhibition of HPV-positive, but not HPV-negative, cervical carcinoma cells, but exerts no such effects on other epithelial tumor cells. Increased Notch1 signaling, but not Notch2, causes a dramatic down-modulation of HPV-driven transcription of the E6/E7 viral genes, through suppression of AP-1 activity by up-regulation of the Fra-1 family member and decreased c-Fos expression. Thus, Notch1 exerts specific protective effects against HPV-induced transformation through suppression of E6/E7 expression, and down-modulation of Notch1 expression is likely to play an important role in late stages of HPV-induced carcinogenesis.
Figures
Figure 1
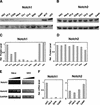
Specific down-modulation of endogenous Notch1 expression in cervical carcinoma cells. (A,B) Total cell extracts from primary human foreskin keratinocytes (hKC), a panel of cervical carcinoma cell lines, cervical keratinocytes (cKC), and a keratinocyte cell line with episomal HPV (W12) were analyzed by immunoblotting with antibodies against Notch1 (C-20) and Notch2 (C65) as indicated (upper panels). Immunoblotting with antibodies against Cdk2 was used as an equal loading control (lower panels). Similar results were obtained in at least two other experiments, including use of Tan-20 antibodies for detection of Notch1, and of anti-β actin antibodies for an equal loading control. (C,D) Densitometric scanning of the autoradiographs shown in A and B was used for quantification of the relative levels of Notch1 and Notch2 proteins in human primary keratinocytes versus the other cells. Cdk2 expression was used for internal value normalization. (E) Total RNA from human primary keratinocytes and HeLa cells was analyzed by RT-PCR with oligonucleotide primers specific for the 3′-untranslated regions of the Notch1 and Notch2 transcript and for GAPDH as indicated. Each sample was analyzed in three serial dilutions (1:1, 1:10, 1:100). (F) Total RNA from human primary keratinocytes and various carcinoma cells was analyzed by real-time RT-PCR with primers specific for the 3′-untranslated regions of the Notch1 and Notch2 transcripts as indicated.Values were normalized for β-actin expression and expressed as relative levels in reference to primary human keratinocytes. All RNAs were tested in triplicate samples, and the standard deviation is indicated.
Figure 2
Down-modulation of Notch1 protein expression in cervical carcinomas. (A) Total cell extracts derived from 293 cells transfected with plasmid expression vectors for Notch1 (lane 1), Notch2 (lane 2), or Notch3 (lane 3) were immunoblotted with the indicated antibodies as control of their specificity. (B) Extracts from surgically excised invasive cervical carcinomas (Carc.1 and Carc.2) and surrounding normal cervical tissues from the same patients (Norm.1 and Norm.2) were analyzed for levels of Notch1 and Notch2 protein expression by immunoblotting with the corresponding antibodies as indicated. Immunoblotting with antibodies against β-actin was used for an equal loading control. (C) Densitometric scanning of the autoradiographs shown in B was used for quantification of the relative levels of Notch1 and Notch2 proteins after normalization for levels of β-actin expression.
Figure 3
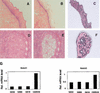
Down-modulation of Notch1 mRNA expression in cervical carcinomas as assessed by laser capture microscopy and real-time RT-PCR. (A_–_F) Histological slides of normal cervical tissue (A_–_C) and an invasive cervical tumor (D_–_F) photographed before laser capture microscopy (A,D) and after removal of the normal (B) and neoplastic (E) epithelium. (C,F) Cap images of the corresponding captured epithelia. Hematoxylin and eosin, ×40. (G) Total RNAs from normal squamous epithelium and three invasive squamous carcinomas of the cervix microdissected by the above technique were analyzed in triplicate samples for levels of Notch1 and Notch2 mRNA expression by real-time RT-PCR as described in Materials and Methods.
Figure 4
Localization of endogenous Notch1 protein expression in normal squamous epithelium and invasive squamous carcinoma of the cervix. (A,B) Serial sections of normal squamous epithelium of the cervix stained with affinity-purified nonimmune antiserum (A) or Tan-20 antibodies against Notch1 (B). (C,D) Staining with Tan-20 antibodies of an area of cervical intraepithelial neoplastic lesion (C) or invasive cervical carcinoma (D) present in the same histological slide as the normal squamous epithelium shown in B. Note the positive staining for Notch1 expression in cells at the transition between normal and neoplastic epithelium (C, arrows). In contrast, little or no Notch1 staining is detectable in the invasive tumor area (D). Similarly, inconspicuous staining with Notch1 antibodies was also found in 10 other advanced cervical cancers that were analyzed. Bar, 80 μm.
Figure 5
Specific growth inhibitory effects of activated Notch1 in cervical carcinoma cell lines. (A) Epidermoid carcinoma (A-431), breast (MDA-MB-453), colon (HCT116), and indicated cervical carcinoma cells were infected with a recombinant adenovirus expressing activated Notch1 (Ad-Notch1) or green fluorescence protein (Ad-GFP). (Upper panel) Total cell extracts from the indicated cell lines infected with the Ad-Notch1 virus were examined for levels of activated Notch1 expression by immunoblotting with the corresponding antibody. (Lower panel) Cellular DNA synthesis was determined by 3H-thymidine incorporation assay 36 h after infection. Cells were tested in triplicated wells, and the standard deviation is indicated. The slight differences in sensitivity of the various HPV-positive carcinoma cell lines to Ad-Notch1 growth inhibition are likely owing to the different growth patterns of these various cells. In fact, some of these cell lines tend to grow in tightly packed clusters, with cells at the center of these clusters being less susceptible to adenoviral infection (as evaluated by expression of the GFP marker, which is also transduced by the Ad-Notch1 virus). (B) HeLa cells were transfected with a plasmid expression vector for activated Notch1 (Notch1ICD) or empty vector control (Ctr) together with trace amounts of a GFP expression vector for identification of transfected cells. Cells were labeled with BrdU for 3 h prior to termination of the experiment (36 h after transfection). The BrdU-labeling index of GFP-positive transfected cells was determined by counting in each case a minimum of 120 cells from six independent fields. Values are expressed as percentages relative to the control. Similar results were obtained in two other independent experiments. (C) HeLa cells were transfected with a plasmid expression vector for activated Notch1 (Notch1ICD) or empty vector control (Ctr) together with trace amounts of an expression vector for G418 resistance. The same number of transfected cells was plated and cultured in triplicate dishes in the presence of antibiotic selection for 2 wk. The number of macroscopically visible colonies formed by cells transfected with the control versus activated Notch1 expression vector was 311 (±20) and 68 (±8), respectively. Similar differences were observed in two other independent experiments. (D) HeLa cells at 24 h after transfection with the activated Notch1 vector (Notch1ICD), and cells from six independent colonies (1–6) that emerged from cultures transfected with the same vector after 2 wk of G418 selection (as in C), were analyzed for levels of Notch1 expression by immunoblotting with the corresponding specific antibodies. (Ctr) Cells from a colony of HeLa cells transfected with empty vector control. The immunoblot was reprobed with anti-cdk2 antibody for an equal loading control.
Figure 6
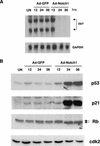
Suppression of E6/E7 mRNA expression by activated Notch1 and associated downstream effects. (A) HeLa cells were either uninfected (UN) or infected with the Ad-Notch1 and Ad-GFP virus for the indicated times (in hours). Levels of HPV18 E6/E7 mRNA expression were determined by Northern blot analysis with corresponding specific probes. Densitomeric scanning of the autoradiographs indicated that activated Notch1 expression caused a 95-fold reduction of E6/E7 mRNA levels by 24 h of infection (i.e., by the time the activated Notch1 protein is expressed). (B) HeLa cells were infected as in A, followed by immunoblot analysis of total cell extracts with antibodies against the p53, p21, and p105-Rb proteins as indicated. Immunoblotting with antibodies against Cdk2 was used for an equal loading control.
Figure 7
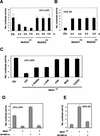
Transcriptional repression of HPV-URR promoter activity by activated Notch1. (A,B) HeLa cells were transfected with either the HPV-URR (A) or HES-AB (B) luciferase reporter plasmids plus/minus expression vectors for the activated cytoplasmic forms of Notch1 and Notch2 in increasing amounts as indicated. Luciferase activity was determined 48 h after transfection and normalized with a Renella reporter internal control. Values are expressed as arbitrary units. All conditions were tested in triplicate samples, and the standard deviation is indicated. (C) HeLa cells were transfected with the HPV-URR reporter plus/minus expression vectors for cytoplasmic activated Notch1 in an intact form (ICD) or its separate (NH2) N- (amino acids 1752–2123) and (COOH) C-terminal (amino acids 2124–2555) regions, or with internal deletions of the (ΔRAM23) RAM23 (amino acids 1751–1851) or (ΔANK) ANK (amino acids 1851–2096) domains, or (ANK) a vector expressing the ANK domain of Notch1 (amino acids 1852–2196) devoid of any other domains. Luciferase activity was determined 48 h after transfection as in A. (D) HeLa cells were transfected with either the HPV-URR (left panel) or HES-AB (right panel) luciferase reporter plasmids plus/minus the expression vector for activated Notch1 (0.90 μg) alone or together with an expression vector for dominant negative RBP-Jκ (0.90 μg) as indicated. Luciferase activity was determined 48 h after transfection as in A.
Figure 8
Suppression of HPV-URR promoter activity by activated Notch1 through differential modulation of specific AP-1 components. (A,B) HeLa cells were transfected with an AP-1 reporter plasmid plus/minus an expression vector for activated Notch1 or empty vector control. Promoter activity was measured under (A) basal conditions and (B) at 12 h of AP-1 activation by phorbol ester TPA treatment (100 ng/mL). (C) HeLa cells were infected with the Ad-GFP or Ad-Notch1 viruses for the indicated times (in hours), and total cell extracts were analyzed by immunoblotting with antibodies against various AP-1 components as indicated. Immunoblotting with antibodies against Cdk2 was used as an equal loading control. Densitometric scanning of the autoradiographs indicated that, relative to the control, expression of Fra-1 was increased 3.2- and 8.5-fold at 24 and 36 h, respectively, after infection with the Ad-Notch1 virus. Conversely, expression of c-Fos was decreased >10-fold at 24 h and 36 h after Ad-Notch1 infection. (D) HeLa cells were transfected with either the pCDNA3 or Notch1ICD plasmids plus/minus the expression vector for dominant-negative RBP-Jκ as indicated. Transient transfection efficiency, as assessed by expression of a GFP expression vector added in trace amounts, was >80%. At 48 h after transfection, total cell extracts were analyzed by immunoblotting with antibodies against Fra-1 or β-actin as indicated. (E) Total extracts from surgically excised cervical carcinomas and corresponding surrounding normal regions (examined for levels of Notch1 and Notch2 expression in Fig. 2B) were analyzed by immunoblotting with antibodies against Fra-1 or β-actin as indicated. (F,G) HeLa cells were transfected with the HPV-URR reporter plus/minus expression vectors for activated Notch1 and c-Fos (F) or anti-sense Fra-1 cDNA (G) in various amounts (in micrograms) as indicated. Luciferase activity was determined at 48 h after infection as in Fig. 7.
Similar articles
- Constitutively active Notch1 induces growth arrest of HPV-positive cervical cancer cells via separate signaling pathways.
Talora C, Cialfi S, Segatto O, Morrone S, Kim Choi J, Frati L, Paolo Dotto G, Gulino A, Screpanti I. Talora C, et al. Exp Cell Res. 2005 May 1;305(2):343-54. doi: 10.1016/j.yexcr.2005.01.015. Exp Cell Res. 2005. PMID: 15817159 - Notch1 can contribute to viral-induced transformation of primary human keratinocytes.
Lathion S, Schaper J, Beard P, Raj K. Lathion S, et al. Cancer Res. 2003 Dec 15;63(24):8687-94. Cancer Res. 2003. PMID: 14695182 - [Possible role of transcription factor AP1 in the tissue-specific regulation of human papillomavirus].
Velazquez Torres A, Gariglio Vidal P. Velazquez Torres A, et al. Rev Invest Clin. 2002 May-Jun;54(3):231-42. Rev Invest Clin. 2002. PMID: 12183893 Review. Spanish. - The role of steroid contraceptive hormones in the pathogenesis of invasive cervical cancer: a review.
Moodley M, Moodley J, Chetty R, Herrington CS. Moodley M, et al. Int J Gynecol Cancer. 2003 Mar-Apr;13(2):103-10. doi: 10.1046/j.1525-1438.2003.13030.x. Int J Gynecol Cancer. 2003. PMID: 12657108 Review.
Cited by
- Notch1 Drives the Formation and Proliferation of Intrahepatic Cholangiocarcinoma.
Guo J, Fu W, Xiang M, Zhang Y, Zhou K, Xu CR, Li L, Kuang D, Ye F. Guo J, et al. Curr Med Sci. 2019 Dec;39(6):929-937. doi: 10.1007/s11596-019-2125-0. Epub 2019 Dec 16. Curr Med Sci. 2019. PMID: 31845224 - Integrated genomic and transcriptional profiling identifies chromosomal loci with altered gene expression in cervical cancer.
Wilting SM, de Wilde J, Meijer CJ, Berkhof J, Yi Y, van Wieringen WN, Braakhuis BJ, Meijer GA, Ylstra B, Snijders PJ, Steenbergen RD. Wilting SM, et al. Genes Chromosomes Cancer. 2008 Oct;47(10):890-905. doi: 10.1002/gcc.20590. Genes Chromosomes Cancer. 2008. PMID: 18618715 Free PMC article. - Human papilloma virus-dependent HMGA1 expression is a relevant step in cervical carcinogenesis.
Mellone M, Rinaldi C, Massimi I, Petroni M, Veschi V, Talora C, Truffa S, Stabile H, Frati L, Screpanti I, Gulino A, Giannini G. Mellone M, et al. Neoplasia. 2008 Aug;10(8):773-81. doi: 10.1593/neo.08462. Neoplasia. 2008. PMID: 18670638 Free PMC article. - Nickel-induced down-regulation of ΔNp63 and its role in the proliferation of keratinocytes.
Zhang Z, Li W, Cheng S, Yao H, Zhang F, Chang Q, Ke Z, Wang X, Son YO, Luo J, Shi X. Zhang Z, et al. Toxicol Appl Pharmacol. 2011 Jun 15;253(3):235-43. doi: 10.1016/j.taap.2011.03.024. Epub 2011 Apr 3. Toxicol Appl Pharmacol. 2011. PMID: 21466819 Free PMC article. - Cellular senescence checkpoint function determines differential Notch1-dependent oncogenic and tumor-suppressor activities.
Kagawa S, Natsuizaka M, Whelan KA, Facompre N, Naganuma S, Ohashi S, Kinugasa H, Egloff AM, Basu D, Gimotty PA, Klein-Szanto AJ, Bass AJ, Wong KK, Diehl JA, Rustgi AK, Nakagawa H. Kagawa S, et al. Oncogene. 2015 Apr 30;34(18):2347-59. doi: 10.1038/onc.2014.169. Epub 2014 Jun 16. Oncogene. 2015. PMID: 24931169 Free PMC article.
References
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. - PubMed
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
- Daniel B, Rangarajan A, Mukherjee G, Vallikad E, Krishna S. The link between integration and expression of human papillomavirus type 16 genomes and cellular changes in the evolution of cervical intraepithelial neoplastic lesions. J Gen Virol. 1997;78:1095–1101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR39190/AR/NIAMS NIH HHS/United States
- CA16038/CA/NCI NIH HHS/United States
- R01 CA073796/CA/NCI NIH HHS/United States
- R01 AR039190/AR/NIAMS NIH HHS/United States
- P01 CA016038/CA/NCI NIH HHS/United States
- CA73796/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous