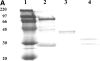I-PLA(2) activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation - PubMed (original) (raw)
I-PLA(2) activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation
Sun Jun Kim et al. J Exp Med. 2002.
Abstract
Deficiency of serum immunoglobulin (Ig)M is associated with the development of a lupus-like disease in mice. Recent studies suggest that classical complement components facilitate the clearance of apoptotic cells and that failure to do so predisposes mice to lupus. Since IgM is a potent activator of the classical complement pathway, we examined IgM binding to dying cells. IgM, but not IgG, bound to apoptotic T cells through the Fab' portion of the antibody. Exposure of apoptotic cell membranes to phospholipase (PL) A2 increased, whereas PLD reduced, IgM binding and complement activation. Absorption studies combined with direct plate binding assays, revealed that IgM antibodies failed to bind to phosphatidyl lipids, but did recognize lysophosphatidylcholine and the phosphorylcholine head group. Both iPLA(2) and cPLA(2) are activated during apoptosis. Since inhibition of iPLA2, but not cPLA2, attenuated IgM binding to apoptotic cells, these results strongly suggest that the endogenous calcium independent PLA(2), iPLA(2), is involved in the hydrolysis of plasma membrane phospholipids and exposure of the epitope(s) recognized by IgM. We propose that recognition of dying cells by natural IgM antibodies is, in part, responsible for complement activation on dying cells leading to their safe clearance.
Figures
Figure 1.
IgM activates complement on apoptotic cells. (A) Normal peripheral blood–derived T cells (PBT) were either incubated in medium or induced to undergo apoptosis by staurosporine (STS) as described in Materials and Methods. The cells were then incubated with either purified human IgG (10 mg/ml) or IgM (1 mg/ml) for 20 min at 37°C and examined for Ig binding by flow cytometry (thin line, live cells; thick line, apoptotic cells; dotted line, apoptotic cells incubated with second antibody alone). The change in mean channel fluorescence (Δ) between the cells incubated with second antibody alone versus IgG or IgM followed by second antibody is shown. Representative of five experiments. (B) Apoptotic PBT cells were incubated with either HGS or autologous NHS for 30 min followed by two-color flow cytometric analysis of IgM and C3b/bi binding. Representative of three experiments. (C) Apoptotic PBT cells were incubated with either 20% autologous NHS or HGS no. 2 and 3 for 30 min at 37°C. Deposition of cell surface C3b/bi and the MAC were quantified by flow cytometry as described in Materials and Methods. As a control for complement activity in the HGS sera, C3b/bi, and MAC deposition on T cells were quantified after incubation with an IgM monoclonal anti-β2 microglobulin antibody followed by either NHS or HGS as a source of complement. The results are expressed as complement component binding in HGS/complement component binding in NHS ×100 (mean ± SD of 4 experiments). (D) C57BL/6 (B6) thymocytes were incubated with 1 μM dexamethasone for 6 h to induce apoptosis. Apoptotic thymocytes were incubated with 20% of autologous wild-type serum or serum obtained from sIgM−/− deficient mice on a B6 or 129 background for 30 min at 37°C. C3 binding to the cells was quantified by flow cytometry. As a control, C3 deposition was also quantified following preincubation of the apoptotic cells with IgM anti-Thy.1 antibodies. The results are expressed as percentage of complement activation. Two experiments gave virtually identical results. (E) Apoptotic PBT cells prepared as in C, were incubated with NHS, HGS, or HGS reconstituted with purified human 10 mg/ml IgG or 1 mg/ml IgM. C3b/bi deposition was detected by flow cytometry and are expressed as the percentage of cells positive for staining (mean ± SD of 3 experiments).
Figure 2.
IgM recruits C1q to apoptotic cells leading to C3 activation. (A) Live or apoptotic PBT cells were incubated with medium alone (−), NHS, HGS, or HGS reconstituted with IgM (1 mg/ml) as indicated for 30 min at 37°C. The cells were washed and C1q binding detected by Western blot analysis as described in Materials and Methods. For Western blot analysis, protein loading was compared by probing the same membrane with anti-ribosomal P antiserum (38 kD). Representative of three experiments. (B) Apoptotic PBT cells were incubated with purified human IgM (1 mg/ml) as above, washed and then incubated with purified human C1q (10 μg/ml) for 20 min at 37°C. C1q binding on the surface of apoptotic cells was detected by flow cytometry using a monoclonal anti–human C1q antibody. The results are expressed as the mean ± SD of three experiments. (C) Apoptotic PBT cells were incubated as in A. with either NHS, HGS, or HGS to which IgM was added to the final concentrations (μg/ml) indicated. These concentrations correspond to serial 10-fold dilutions of the normal serum concentration (1 mg/ml). Parallel experiments were performed with C1q depleted serum (C1q-D) to which serial 10-fold dilutions of C1q were added (normal serum concentration is 50 μg/ ml). C3b/bi binding was detected by flow cytometry and expressed as the percentage of cells positive for staining as in Fig. 1 E (mean ± SD of 3 experiments).
Figure 2.
IgM recruits C1q to apoptotic cells leading to C3 activation. (A) Live or apoptotic PBT cells were incubated with medium alone (−), NHS, HGS, or HGS reconstituted with IgM (1 mg/ml) as indicated for 30 min at 37°C. The cells were washed and C1q binding detected by Western blot analysis as described in Materials and Methods. For Western blot analysis, protein loading was compared by probing the same membrane with anti-ribosomal P antiserum (38 kD). Representative of three experiments. (B) Apoptotic PBT cells were incubated with purified human IgM (1 mg/ml) as above, washed and then incubated with purified human C1q (10 μg/ml) for 20 min at 37°C. C1q binding on the surface of apoptotic cells was detected by flow cytometry using a monoclonal anti–human C1q antibody. The results are expressed as the mean ± SD of three experiments. (C) Apoptotic PBT cells were incubated as in A. with either NHS, HGS, or HGS to which IgM was added to the final concentrations (μg/ml) indicated. These concentrations correspond to serial 10-fold dilutions of the normal serum concentration (1 mg/ml). Parallel experiments were performed with C1q depleted serum (C1q-D) to which serial 10-fold dilutions of C1q were added (normal serum concentration is 50 μg/ ml). C3b/bi binding was detected by flow cytometry and expressed as the percentage of cells positive for staining as in Fig. 1 E (mean ± SD of 3 experiments).
Figure 2.
IgM recruits C1q to apoptotic cells leading to C3 activation. (A) Live or apoptotic PBT cells were incubated with medium alone (−), NHS, HGS, or HGS reconstituted with IgM (1 mg/ml) as indicated for 30 min at 37°C. The cells were washed and C1q binding detected by Western blot analysis as described in Materials and Methods. For Western blot analysis, protein loading was compared by probing the same membrane with anti-ribosomal P antiserum (38 kD). Representative of three experiments. (B) Apoptotic PBT cells were incubated with purified human IgM (1 mg/ml) as above, washed and then incubated with purified human C1q (10 μg/ml) for 20 min at 37°C. C1q binding on the surface of apoptotic cells was detected by flow cytometry using a monoclonal anti–human C1q antibody. The results are expressed as the mean ± SD of three experiments. (C) Apoptotic PBT cells were incubated as in A. with either NHS, HGS, or HGS to which IgM was added to the final concentrations (μg/ml) indicated. These concentrations correspond to serial 10-fold dilutions of the normal serum concentration (1 mg/ml). Parallel experiments were performed with C1q depleted serum (C1q-D) to which serial 10-fold dilutions of C1q were added (normal serum concentration is 50 μg/ ml). C3b/bi binding was detected by flow cytometry and expressed as the percentage of cells positive for staining as in Fig. 1 E (mean ± SD of 3 experiments).
Figure 3.
IgM binds to apoptotic cells by its Fab domain. Fc and Fab fragments of IgM were prepared by tryptic digestion and were isolated by FPLC as described in Materials and Methods. (A) Purity of the fragments (20 μg per lane) was assessed by SDS -10% PAGE under reducing conditions and proteins detected by Commassie-Blue staining. Lane 1, molecular mass standards; lane 2, IgM before digestion; lane 3, Fcμ; lane 4, Fab′. (B) Isolated IgM, IgM heated to 65°C for 10 min, IgM Fcμ or Fab′ fragments (all tested at a concentration of 1 pM) were examined for binding to apoptotic PBT as in Fig. 1 except that IgM Fc or Fab′ binding were detected with polyclonal antibodies specific for the fragment. The results are expressed as the mean ± SD of four experiments.
Figure 3.
IgM binds to apoptotic cells by its Fab domain. Fc and Fab fragments of IgM were prepared by tryptic digestion and were isolated by FPLC as described in Materials and Methods. (A) Purity of the fragments (20 μg per lane) was assessed by SDS -10% PAGE under reducing conditions and proteins detected by Commassie-Blue staining. Lane 1, molecular mass standards; lane 2, IgM before digestion; lane 3, Fcμ; lane 4, Fab′. (B) Isolated IgM, IgM heated to 65°C for 10 min, IgM Fcμ or Fab′ fragments (all tested at a concentration of 1 pM) were examined for binding to apoptotic PBT as in Fig. 1 except that IgM Fc or Fab′ binding were detected with polyclonal antibodies specific for the fragment. The results are expressed as the mean ± SD of four experiments.
Figure 4.
Kinetics and specificity of IgM binding to apoptotic cells. (A) Apoptosis of PBT cells was induced as in Fig. 1 and at time 0, 2, 4 and 6 h, the cells were incubated in medium containing 20% NHS or HGS. Cells were analyzed by flow cytometry for Annexin V or IgM binding as well as for permeability to PI and trypan blue as indicated in the Figure. The results are expressed as the percentage of cells positive. The results are expressed as the mean ± SD of three experiments. (B) Purified IgM was incubated with liposomes containing either 50 or 500 ug/ml PtS or PtC for 30 min at 37°C. Samples were centrifuged and the supernates tested for binding to apoptotic cells. Annexin V and SUV (an anti-PtC specific mAb), were used as positive controls for binding to PtS and PtC, respectively. The results are expressed as percentage of inhibition of binding, calculated from (binding in medium − binding after preadsorbtion with liposome/binding in medium) × 100. The results are expressed as the mean ± SD of three experiments.
Figure 5.
IgM antibodies bind to components of lysophospholipids on the cell surface membrane. (A) Apoptotic cells were incubated in medium alone or medium containing 1 U of PLs (PLA2 or PLD) for 30 min at 37°C or on ice (0°C). The cells were washed and then incubated with purified IgM or the TEPC15 anti-PC mAb for 20 min at 37°C. IgM binding to live cells is shown for comparison. Antibody binding was detected by flow cytometry and is expressed as the mean ± SD of 4 experiments. (B) Thymocytes were rendered apoptotic as in Fig. 1 and then exposed to 1U of type I or type III sPLA2 or 1U PLD for 30 min at 37°C. They were then washed and incubated with wild-type (WT) or IgM-deficient (sIgM−/−) serum followed by flow cytometry analysis for C3 binding. The results are expressed as the percentage of cells staining for C3 and are representative of two experiments. Cells incubated with heat-inactivated serum (HIS) or anti-Thy1 were used as negative and positive controls, respectively. (C) NHS was incubated with solid phase adsorbed lysophospholipids and then tested for binding to apoptotic cells. The results are expressed as inhibition of IgM binding, calculated as in Fig. 4 and are the mean ± SD of 3 experiments. (D) NHS was preincubated with varying concentration of PC, PS, or PE as shown for 30 min at 37°C. Inhibition of IgM binding to apoptotic cells was calculated as above. The results are expressed as the mean ± SD of 3 experiments. (E) Purified IgM or NHS was incubated with PC (PC-Cl) and the percentage inhibition of IgM binding to apoptotic cells determined by flow cytometry as in C. TEPC15, and an anti-β2 microglobulin mAb, were used as positive and negative controls, respectively. The results are expressed as the mean ± SD of 4 experiments.
Figure 6.
BEL, an inhibitor of endogenous iPLA2 attenuates IgM binding to apoptotic cells. (A) PBMT were incubated with staurosporine together with medium alone or medium containing the PLA2 inhibitors, 10 μM BEL or 5 μM Shionogi-1 for 6 h (A and C) or the phosphatidate phosphohydrolase inhibitor, propranolol (12.5 μM) (B). The cells were then stained with Annexin V-FITC (A and B) and/or purified human IgM followed by FITC-anti–human IgM (B). To determine the effects of BEL on intracellular apoptotic events, cells were examined for caspase-3 activity by flow cytometry (C), PARP cleavage by Western blot analysis (D), or nuclear condensation by Hoechst staining and immunofluorescence (E) in the presence or absence of 10 μM BEL as described in Materials and Methods. For Western blot analysis (D), protein loading was compared by probing the same membrane with antiribosomal P antiserum (38 kD). A–C are mean ± SD of 3 experiments; D and E are representative of two experiments with identical results.
Figure 7.
Proposed role of IgM in the binding and clearance of apoptotic cells. Apoptotic cells activate iPLA2, which results in exposure of lysophospholipids, including lyso-PtC, on the cell membrane. Under normal circumstances, lysoPtC is recognized by IgM which activates the classical complement pathway (A). As shown, not all complement activation is IgM dependent. Macrophages or dendritic cells phagocytose complement-coated cells, and produce immunosuppressive cytokines such as TGF-β (see references 50, 6, and ; A). In contrast, when little IgM is available (B), either the cells undergo post-apoptotic necrosis and/or are seen by IgG antibodies. In either case, phagocytosis of this cargo leads to proinflammatory cytokine production (see references 50, and 6) and, possibly, maturation of dendritic cells (reference 55).
Similar articles
- Annexin A2 and A5 serve as new ligands for C1q on apoptotic cells.
Martin M, Leffler J, Blom AM. Martin M, et al. J Biol Chem. 2012 Sep 28;287(40):33733-44. doi: 10.1074/jbc.M112.341339. Epub 2012 Aug 9. J Biol Chem. 2012. PMID: 22879587 Free PMC article. - Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro.
Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Quartier P, et al. Eur J Immunol. 2005 Jan;35(1):252-60. doi: 10.1002/eji.200425497. Eur J Immunol. 2005. PMID: 15597324 - Direct binding of C1q to apoptotic cells and cell blebs induces complement activation.
Nauta AJ, Trouw LA, Daha MR, Tijsma O, Nieuwland R, Schwaeble WJ, Gingras AR, Mantovani A, Hack EC, Roos A. Nauta AJ, et al. Eur J Immunol. 2002 Jun;32(6):1726-36. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R. Eur J Immunol. 2002. PMID: 12115656 - Calcium-independent phospholipase A2 and apoptosis.
Balsinde J, Pérez R, Balboa MA. Balsinde J, et al. Biochim Biophys Acta. 2006 Nov;1761(11):1344-50. doi: 10.1016/j.bbalip.2006.07.013. Epub 2006 Aug 3. Biochim Biophys Acta. 2006. PMID: 16962822 Review. - The role of IgM antibodies in the recognition and clearance of apoptotic cells.
Peng Y, Kowalewski R, Kim S, Elkon KB. Peng Y, et al. Mol Immunol. 2005 May;42(7):781-7. doi: 10.1016/j.molimm.2004.07.045. Epub 2005 Jan 16. Mol Immunol. 2005. PMID: 15829266 Review.
Cited by
- Defective B cell tolerance checkpoints in systemic lupus erythematosus.
Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Yurasov S, et al. J Exp Med. 2005 Mar 7;201(5):703-11. doi: 10.1084/jem.20042251. Epub 2005 Feb 28. J Exp Med. 2005. PMID: 15738055 Free PMC article. - Regulation of interleukin-10 gene expression in macrophages engulfing apoptotic cells.
Zhang Y, Kim HJ, Yamamoto S, Kang X, Ma X. Zhang Y, et al. J Interferon Cytokine Res. 2010 Mar;30(3):113-22. doi: 10.1089/jir.2010.0004. J Interferon Cytokine Res. 2010. PMID: 20187777 Free PMC article. Review. - Specific binding of an antigen-antibody complex to apoptotic human neutrophils.
Hart SP, Jackson C, Kremmel LM, McNeill MS, Jersmann H, Alexander KM, Ross JA, Dransfield I. Hart SP, et al. Am J Pathol. 2003 Mar;162(3):1011-8. doi: 10.1016/S0002-9440(10)63895-3. Am J Pathol. 2003. PMID: 12598333 Free PMC article. - The Sound of Silence: Signaling by Apoptotic Cells.
Fogarty CE, Bergmann A. Fogarty CE, et al. Curr Top Dev Biol. 2015;114:241-65. doi: 10.1016/bs.ctdb.2015.07.013. Epub 2015 Sep 11. Curr Top Dev Biol. 2015. PMID: 26431570 Free PMC article. Review. - Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention.
Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Dennis EA, et al. Chem Rev. 2011 Oct 12;111(10):6130-85. doi: 10.1021/cr200085w. Epub 2011 Sep 12. Chem Rev. 2011. PMID: 21910409 Free PMC article. Review. No abstract available.
References
- Wakeland, E.K., K. Liu, R.R. Graham, and T.W. Behrens. 2001. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 15:397–408. - PubMed
- Scott, R.S., E.J. McMahon, S.M. Pop, E.A. Reap, R. Caricchio, P.L. Cohen, H.S. Earp, and G.K. Matsushima. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 411:207–211. - PubMed
- Napirei, M., H. Karsunky, B. Zevnik, H. Stephan, H.G. Mannherz, and T. Moroy. 2000. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat. Genet. 25:177–181. - PubMed
- Botto, M., C. Dell'Agnola, A.E. Bygrave, E.M. Thompson, T. Cook, F. Petry, M. Loos, P.P. Pandolfi, and M.J. Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Gen. 19:56–59. - PubMed
- Bickerstaff, M.C.M., M. Botto, W.L. Hutchinson, J. Herbert, G.A. Tennent, A. Bybee, D.A. Mitchell, H.T. Cook, P.J.G. Butler, M.J. Walport, et al. 1999. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat. Med. 5:694–697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases