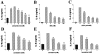Hyperosmotic stress induces nuclear factor-kappaB activation and interleukin-8 production in human intestinal epithelial cells - PubMed (original) (raw)
Hyperosmotic stress induces nuclear factor-kappaB activation and interleukin-8 production in human intestinal epithelial cells
Zoltán H Németh et al. Am J Pathol. 2002 Sep.
Abstract
Inflammatory bowel disease of the colon is associated with a high osmolarity of colonic contents. We hypothesized that this hyperosmolarity may contribute to colonic inflammation by stimulating the proinflammatory activity of intestinal epithelial cells (IECs). The human IEC lines HT-29 and Caco-2 were used to study the effect of hyperosmolarity on the IEC inflammatory response. Exposure of IECs to hyperosmolarity triggered expression of the proinflammatory chemokine interleukin (IL)-8 both at the secreted protein and mRNA levels. In addition, hyperosmotic stimulation induced the release of another chemokine, GRO-alpha. These effects were because of activation of the transcription factor, nuclear factor (NF)-kappaB, because hyperosmolarity stimulated both NF-kappaB DNA binding and NF-kappaB-dependent transcriptional activity. Hyperosmolarity activated both p38 and p42/44 mitogen-activated protein kinases, which effect contributed to hyperosmolarity-stimulated IL-8 production, because p38 and p42/44 inhibition prevented the hyperosmolarity-induced increase in IL-8 production. In addition, the proinflammatory effects of hyperosmolarity were, in a large part, mediated by activation of Na(+)/H(+) exchangers, because selective blockade of Na(+)/H(+) exchangers prevented the hyperosmolarity-induced IEC inflammatory response. In summary, hyperosmolarity stimulates IEC IL-8 production, which effect may contribute to the maintenance of inflammation in inflammatory bowel disease.
Figures
Figure 1.
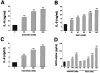
Hyperosmolarity stimulates IL-8 production by both HT-29 (A and B) and Caco-2 (C) cells. Cells were incubated with hyperosmotic medium prepared by the addition of mannitol (A and C) or NaCl (B) to isosmolar growth medium. NaCl concentrations depicted on the x axis represent NaCl amounts added to isosmolar growth medium that already contains ∼140 mmol/L of NaCl (B). In control wells, cells were incubated with isosmolar growth medium. D: Hyperosmotic medium containing either mannitol or NaCl also stimulates GRO-α production by HT-29 cells. Supernatants for IL-8 and GRO-α measurement were taken 18 hours after the hyperosmolar challenge. Data are mean ± SEM of n = 6 to 12 wells from two different experiments. *, P < 0.05; **, P < 0.01.
Figure 2.
Treatment of HT-29 cells with the selective NHE inhibitors amiloride (A), MIA (B), or EIPA (C) suppresses mannitol-induced IL-8 production. Treatment of HT-29 cells with the nonamiloride NHE inhibitors cimetidine (D), clonidine (E), or harmaline (F), reproduces the suppressive effect of selective NHE inhibitors on the production of IL-8 by mannitol-induced HT-29 cells. When harmaline was used, supernatants for IL-8 measurement were taken 4 hours after the hyperosmotic challenge. In the case of amiloride, MIA, EIPA, cimetidine, and clonidine, IL-8 levels were measured from supernatants obtained 18 hours after the hyperosmotic challenge. Hyperosmotic medium prepared by the addition of 100 mmol/L of mannitol to isosmolar growth medium was used for hyperosmotic stimulation. Data are mean ± SEM of n = 6 to 12 wells from two different experiments. *, P < 0.05; **, P < 0.01. Dotted bar, no mannitol; cross-hatched bars, mannitol.
Figure 3.
A: DMSO (0.5%) inhibits both basal and mannitol (100 mmol/L)-stimulated IL-8 production by HT-29 cells. Growth medium on cells was switched with fresh growth medium with or without mannitol, both in the presence or absence of 0.5% DMSO. IL-8 production was measured from supernatants taken 18 hours after the addition of fresh medium. B: Increasing medium pH fails to augment the production of IL-8 by HT-29 cells. Growth medium on cells was switched with fresh growth medium with pH values ranging from 7.3 to 7.8. IL-8 production was measured from supernatants taken 18 hours after the addition of fresh medium. Data are mean ± SEM of n = 6 to 12 wells from two different experiments. **, P < 0.01.
Figure 4.
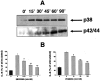
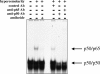
Hyperosmolarity induces p38 and p42/44 activation in HT-29 cells (A). Cells were incubated with hyperosmotic medium prepared by the addition of mannitol (for a final concentration of 100 mmol/L) to isosmolar medium for the indicated time periods. p38 and p42/44 activation was determined using Western blotting using antibodies raised against the active, double-phosphorylated form of p38 and p42/44. This figure is representative of two separate experiments. Treatment of HT-29 cells with the selective p38 inhibitor SB203580 or selective p42/44 inhibitor PD98059 suppresses the hyperosmolarity-induced IL-8 response (B). Hyperosmolarity was achieved by the addition of mannitol (for a final concentration of 100 mmol/L) to isosmolar medium. In control wells, cells were incubated with isosmolar medium. Data are mean ± SEM of n = 12 wells from two separate experiments. *, P < 0.05; **, P < 0.01. Dotted bar, no mannitol; cross-hatched bars, mannitol.
Figure 5.
Hyperosmolarity induces up-regulation of IL-8 mRNA levels in HT-29 cells. Amiloride pretreatment (300 μmol/L) inhibits hyperosmolarity-induced IL-8 mRNA accumulation. Lanes 1 and 2: Control (isosmolar medium); lanes 3 and 4: hyperosmolarity (100 mmol/L of mannitol); lanes 5 and 6: amiloride and hyperosmolarity. GAPDH levels were not affected by both hyperosmolar and amiloride treatment. IL-8 and GAPDH mRNA levels were quantitated using reverse transcriptase-PCR. This figure is representative of three separate experiments.
Figure 6.
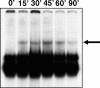
Hyperosmolarity induces NF-κB DNA binding in HT-29 cells. Cells were incubated with hyperosmotic medium prepared by the addition of mannitol (for a final concentration of 100 mmol/L) to isosmolar medium for the indicated time periods. NF-κB DNA binding was assessed using EMSA. The figure is representative of two separate experiments.
Figure 7.
Amiloride pretreatment (300 μmol/L) inhibits hyperosmolarity-induced NF-κB DNA binding in HT-29 cells. NF-κB-specific complexes are indicated by arrows as determined by antibody supershifting. Hyperosmolarity was produced by the addition of mannitol (for a final concentration of 100 mmol/L) to isosmolar medium for 45 minutes. Control cells were treated with isosmolar medium. This figure is representative of three separate experiments.
Figure 8.
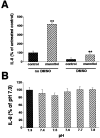
Hyperosmolar stimulation increases NF-κB-dependent transcriptional activity as compared to isosmolar stimulation. HT-29 cells were transiently transfected with a NF-κB-luciferase promoter construct, after which the cells were incubated for 16 hours with either hyperosmolar (100 mmol/L of mannitol) or isosmolar (control) medium. NF-κB-dependent transcriptional activity was determined using the luciferase assay (pNF-κB-Luc). This figure also shows that hyperosmolarity does not influence luciferase activity in cells transfected with an enhancerless construct (pTAL-Luc). Data are mean ± SEM of n = 14 to 16 wells from two separate experiments. **, P < 0.01.
Similar articles
- NF-kappa B is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria.
Elewaut D, DiDonato JA, Kim JM, Truong F, Eckmann L, Kagnoff MF. Elewaut D, et al. J Immunol. 1999 Aug 1;163(3):1457-66. J Immunol. 1999. PMID: 10415047 - The p38 mitogen-activated protein kinase regulates interleukin-1beta-induced IL-8 expression via an effect on the IL-8 promoter in intestinal epithelial cells.
Parhar K, Ray A, Steinbrecher U, Nelson C, Salh B. Parhar K, et al. Immunology. 2003 Apr;108(4):502-12. doi: 10.1046/j.1365-2567.2003.01603.x. Immunology. 2003. PMID: 12667212 Free PMC article. - Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells.
Yang SK, Eckmann L, Panja A, Kagnoff MF. Yang SK, et al. Gastroenterology. 1997 Oct;113(4):1214-23. doi: 10.1053/gast.1997.v113.pm9322516. Gastroenterology. 1997. PMID: 9322516 - Role of CXCL1 in tumorigenesis of melanoma.
Dhawan P, Richmond A. Dhawan P, et al. J Leukoc Biol. 2002 Jul;72(1):9-18. J Leukoc Biol. 2002. PMID: 12101257 Free PMC article. Review.
Cited by
- Hyperosmolarity Triggers the Warburg Effect in Chinese Hamster Ovary Cells and Reveals a Reduced Mitochondria Horsepower.
da Veiga Moreira J, De Staercke L, César Martínez-Basilio P, Gauthier-Thibodeau S, Montégut L, Schwartz L, Jolicoeur M. da Veiga Moreira J, et al. Metabolites. 2021 May 26;11(6):344. doi: 10.3390/metabo11060344. Metabolites. 2021. PMID: 34073567 Free PMC article. - Expression of Inflammatory-Related NFκB Genes in Iranian Patients with Pterygium: A Case-Control Study.
Zaheryani SMS, Ebrahimi ME, Kasaei A, Roointan A, Nejabat M, Dianatpour M, Meisam M, Talebnejad MR, Naghibalhossaini F. Zaheryani SMS, et al. Int J Mol Cell Med. 2018 Summer;7(3):169-175. doi: 10.22088/IJMCM.BUMS.7.3.169. Epub 2018 Oct 22. Int J Mol Cell Med. 2018. PMID: 31565648 Free PMC article. - In Vitro Inhibition of NFAT5-Mediated Induction of CCL2 in Hyperosmotic Conditions by Cyclosporine and Dexamethasone on Human HeLa-Modified Conjunctiva-Derived Cells.
Warcoin E, Baudouin C, Gard C, Brignole-Baudouin F. Warcoin E, et al. PLoS One. 2016 Aug 3;11(8):e0159983. doi: 10.1371/journal.pone.0159983. eCollection 2016. PLoS One. 2016. PMID: 27486749 Free PMC article. - Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis.
Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman SV, Merlin D. Yan Y, et al. PLoS One. 2009 Jun 29;4(6):e6073. doi: 10.1371/journal.pone.0006073. PLoS One. 2009. PMID: 19562033 Free PMC article. - Ste20-related proline/alanine-rich kinase (SPAK) regulated transcriptionally by hyperosmolarity is involved in intestinal barrier function.
Yan Y, Dalmasso G, Nguyen HT, Obertone TS, Sitaraman SV, Merlin D. Yan Y, et al. PLoS One. 2009;4(4):e5049. doi: 10.1371/journal.pone.0005049. Epub 2009 Apr 3. PLoS One. 2009. PMID: 19343169 Free PMC article.
References
- Fiocchi C: Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am J Physiol 1997, 273:G769-G775 - PubMed
- Hecht G, Savkovic SD: Effector role of epithelia in inflammation—interaction with bacteria. Aliment Pharmacol Ther 1997, 3:S64-S68 - PubMed
- Schmid RM, Adler G: NF-kappaB/rel/IkappaB: implications in gastrointestinal diseases. Gastroenterology 2000, 118:1208-1228 - PubMed
- Jobin C, Sartor RB: The Iκ B/NF-κB system: a key determinant of mucosal inflammation and protection. Am J Physiol 2000, 278:C451-C462 - PubMed
- Madara JL: Pathobiology of neutrophil interactions with intestinal epithelia. Aliment Pharmacol Ther 1997, 3:S57-S62 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources