Engineering growing tissues - PubMed (original) (raw)
Engineering growing tissues
Eben Alsberg et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Regenerating or engineering new tissues and organs may one day allow routine replacement of lost or failing tissues and organs. However, these engineered tissues must not only grow to fill a defect and integrate with the host tissue, but often they must also grow in concert with the changing needs of the body over time. We hypothesized that tissues capable of growing with time could be engineered by supplying growth stimulus signals to cells from the biomaterial used for cell transplantation. In this study, chondrocytes and osteoblasts were cotransplanted on hydrogels modified with an RGD-containing peptide sequence to promote cell multiplication. New bone tissue was formed that grew in mass and cellularity by endochondral ossification in a manner similar to normal long-bone growth. Transplanted cells organized into structures that morphologically and functionally resembled growth plates. These engineered tissues could find utility in treating diseases and injuries of the growth plate, testing the effect of experimental drugs on growth-plate function and development, and investigating the biology of long-bone growth. Furthermore, this concept of promoting the growth of engineered tissues could find great utility in engineering numerous tissue types by way of the transplantation of a small number of precursor cells.
Figures
Fig 1.
Growing cartilaginous tissues were engineered with appropriate polymeric delivery vehicles. The gross appearance of chondrocytes transplanted with unmodified alginate (a) and RGD-modified alginate (b) after 25 weeks, and changes in implant mass over time (c) demonstrated growth of the engineered tissue when adhesion ligands are provided by the transplantation vehicle. Masson's Trichrome stain indicating the presence of cross-linked collagen after 6 weeks in the unmodified alginate/chondrocyte group (×100) (d) and the RGD-modified alginate/chondrocyte group (×100) (e), although the extent of formation of a cartilaginous tissue was accelerated in the RGD-alginate vehicle.
Fig 2.
Engineering bony tissues with osteoblast and chondrocyte cotransplantation results in an increased mass, mineral content, and cellularity with time, as contrasted to osteoblast-alone transplantation. Gross appearance and dual-energy x-ray absorptiometric images at 26 weeks of implants resulting from transplantation of RCO cells only (a and c), and a 2:1 ratio of RCO to BAC cells (b and d) in an RGD-modified alginate qualitatively demonstrated increased implant size and mineral content as a result of cotransplantation. Quantification of changes in bone mineral density (e) and bone mineral content (f) indicated significantly greater bone mineral content in the implants containing a 2:1 ratio of BAC cells to RCO cells compared with the condition with RCO cells only at 4 and 26 weeks. Plots of implant mass (g) and total cell number (h) over time revealed significant increases over time only in the cotransplantation group. The mass and total number of cells of the cotransplantation group were significantly greater than the RCO-only group at 26 weeks. Within the cotransplantation group, masses and total number of cells were significantly greater at 26 weeks than at 4 and 13 weeks.
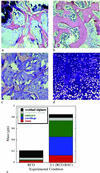
Fig 3.
Tissues composed of both bone and cartilage were engineered through cotransplantation. Hematoxylin/eosin-stained sections demonstrate mature bone formation at 26 weeks in implants consisting of alginate-RGD mixed with RCO cells (a), and a 2:1 ratio of RCO to BAC cells (b), although substantial marrow space was observed only in the cotransplantation group (×100). Aldehyde fuchsin/alcian blue and eosin staining of sections revealed only residual alginate in the RCO-only implants (c). In contrast, an abundant, highly cellular cartilaginous matrix was observed in the 2:1 cotransplantation implants (d) at 26 weeks (×100). Histomorphometric quantification of implant tissue compositions (e) clearly depicts substantial cartilage formation in the cotransplantation group, and significantly more bone and marrow space in these engineered tissues, compared with the RCO-only control.
Fig 4.
Cotransplantation in the RGD-vehicle provided the necessary signals for the formation of growth-plate-like structures. Low magnification of a histologic section indicates the macroscopic organization of the growth-plate-like structures (a) (×20). Examination of the interface between cartilaginous and bony regions of tissues engineered with cotransplanted osteoblasts and chondrocytes demonstrated a structure (b) similar to that seen in developing long bones (×100). Magnification of the cartilage (c), transition (d), and bone and marrow space (e) regions demonstrated cellular and tissue morphology typical of the corresponding regions in a growth plate (×200).
Similar articles
- Tissue-engineered composites of bone and cartilage for mandible condylar reconstruction.
Weng Y, Cao Y, Silva CA, Vacanti MP, Vacanti CA. Weng Y, et al. J Oral Maxillofac Surg. 2001 Feb;59(2):185-90. doi: 10.1053/joms.2001.20491. J Oral Maxillofac Surg. 2001. PMID: 11213987 - Shape-defining scaffolds for minimally invasive tissue engineering.
Thornton AJ, Alsberg E, Albertelli M, Mooney DJ. Thornton AJ, et al. Transplantation. 2004 Jun 27;77(12):1798-803. doi: 10.1097/01.tp.0000131152.71117.0e. Transplantation. 2004. PMID: 15223894 - Tracheal reconstruction using tissue-engineered cartilage.
Grimmer JF, Gunnlaugsson CB, Alsberg E, Murphy HS, Kong HJ, Mooney DJ, Weatherly RA. Grimmer JF, et al. Arch Otolaryngol Head Neck Surg. 2004 Oct;130(10):1191-6. doi: 10.1001/archotol.130.10.1191. Arch Otolaryngol Head Neck Surg. 2004. PMID: 15492167 - Bone and cartilage tissue engineering for facial reconstructive surgery.
Farhadi J, Jaquiery C, Haug M, Pierer G, Zeilhofer HF, Martin I. Farhadi J, et al. IEEE Eng Med Biol Mag. 2006 Jan-Feb;25(1):106-9. doi: 10.1109/memb.2006.1578673. IEEE Eng Med Biol Mag. 2006. PMID: 16485400 Review. No abstract available. - Role of alginate in bone tissue engineering.
Venkatesan J, Nithya R, Sudha PN, Kim SK. Venkatesan J, et al. Adv Food Nutr Res. 2014;73:45-57. doi: 10.1016/B978-0-12-800268-1.00004-4. Adv Food Nutr Res. 2014. PMID: 25300542 Review.
Cited by
- Study on the potential of RGD- and PHSRN-modified alginates as artificial extracellular matrices for engineering bone.
Nakaoka R, Hirano Y, Mooney DJ, Tsuchiya T, Matsuoka A. Nakaoka R, et al. J Artif Organs. 2013 Sep;16(3):284-93. doi: 10.1007/s10047-013-0703-7. Epub 2013 Mar 20. J Artif Organs. 2013. PMID: 23512309 - Bioengineering of injectable encapsulated aggregates of pluripotent stem cells for therapy of myocardial infarction.
Zhao S, Xu Z, Wang H, Reese BE, Gushchina LV, Jiang M, Agarwal P, Xu J, Zhang M, Shen R, Liu Z, Weisleder N, He X. Zhao S, et al. Nat Commun. 2016 Oct 27;7:13306. doi: 10.1038/ncomms13306. Nat Commun. 2016. PMID: 27786170 Free PMC article. - Peptide-based Biopolymers in Biomedicine and Biotechnology.
Chow D, Nunalee ML, Lim DW, Simnick AJ, Chilkoti A. Chow D, et al. Mater Sci Eng R Rep. 2008 Jan;62(4):125-155. doi: 10.1016/j.mser.2008.04.004. Mater Sci Eng R Rep. 2008. PMID: 19122836 Free PMC article. - Engineering alginate as bioink for bioprinting.
Jia J, Richards DJ, Pollard S, Tan Y, Rodriguez J, Visconti RP, Trusk TC, Yost MJ, Yao H, Markwald RR, Mei Y. Jia J, et al. Acta Biomater. 2014 Oct;10(10):4323-31. doi: 10.1016/j.actbio.2014.06.034. Epub 2014 Jul 1. Acta Biomater. 2014. PMID: 24998183 Free PMC article. - Novel 3D co-culture model for epithelial-stromal cells interaction in prostate cancer.
Fang X, Sittadjody S, Gyabaah K, Opara EC, Balaji KC. Fang X, et al. PLoS One. 2013 Sep 20;8(9):e75187. doi: 10.1371/journal.pone.0075187. eCollection 2013. PLoS One. 2013. PMID: 24073251 Free PMC article.
References
- Langer R. & Vacanti, J. P. (1993) Science 260, 920-926. - PubMed
- Putnam A. J. & Mooney, D. J. (1996) Nat. Med. 2, 824-826. - PubMed
- Jee W. S. S. (1987) in Histology: Cell and Tissue Biology, ed. Weiss, L. (Elsevier, New York), pp. 212–254.
- Beers M. H., Berkow, R. & Burs, M., (1999) The Merck Manual of Diagnosis and Therapy (Merck, Rahway, NJ).
- Peppas N. A. & Langer, R. (1994) Science 263, 1715-1720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DE13033/DE/NIDCR NIH HHS/United States
- T32 GM008353/GM/NIGMS NIH HHS/United States
- GM08353/GM/NIGMS NIH HHS/United States
- R01 DE013033/DE/NIDCR NIH HHS/United States
- T32 DC005356/DC/NIDCD NIH HHS/United States
- T32-DC05356/DC/NIDCD NIH HHS/United States
- T32 DE007057/DE/NIDCR NIH HHS/United States
- T32-DE07057/DE/NIDCR NIH HHS/United States
- T32 GM145304/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources