The product of the survival of motor neuron (SMN) gene is a human telomerase-associated protein - PubMed (original) (raw)
The product of the survival of motor neuron (SMN) gene is a human telomerase-associated protein
François Bachand et al. Mol Biol Cell. 2002 Sep.
Abstract
Telomerase is a ribonucleoprotein (RNP) complex that is minimally composed of a protein catalytic subunit, the telomerase reverse transcriptase (TERT), and an RNA component, the telomerase RNA. The survival of motor neuron (SMN) gene codes for a protein involved in the biogenesis of certain RNPs. Here, we report that SMN is a telomerase-associated protein. Using in vitro binding assays and immunoprecipitation experiments, we demonstrate an association between SMN and the telomerase RNP in vitro and in human cells. The specific immunopurification of SMN from human 293 cells copurified telomerase activity, suggesting that SMN associates with a subset of the functional telomerase holoenzyme. Our results also indicate that the human telomerase RNA and the human (h) TERT are not associated with Sm proteins, in contrast to Saccharomyces cerevisiae telomerase. Immunofluorescence analysis showed that hTERT does not specifically colocalize with wild-type SMN in gems or Cajal bodies. However, a dominant-negative mutant of SMN (SMNDeltaN27) previously characterized to elicit the cellular reorganization of small nuclear RNPs caused the accumulation of hTERT in specific SMNDeltaN27-induced cellular bodies. Furthermore, coexpression of SMNDeltaN27 and hTERT in rabbit reticulocyte lysates decreased the efficiency of human telomerase reconstitution in vitro. Our results establish SMN as a novel telomerase-associated protein that is likely to function in human telomerase biogenesis.
Figures
Figure 1
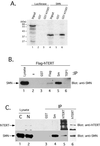
SMN associates with hTERT in vitro and in vivo. (A) Human telomerase was reconstituted by coexpression of GST-hTERT and hTR in S. cerevisiae as previously described (Bachand and Autexier, 1999). GST (lanes 2 and 5) and GST-hTERT/hTR (lanes 3 and 6) were affinity-purified from equal volumes of yeast extracts and incubated with in vitro–translated [35S]methionine-labeled luciferase (lanes 1–3) and SMN (lanes 4–6). After extensive washing, bound proteins were analyzed by SDS-PAGE and autoradiography. The input lanes (1 and 4) show 5% of the RRL lysate used in the binding reaction. Molecular mass markers are indicated on the left (in kilodaltons, kDa). (B) 293 cells were transiently transfected with a DNA construct expressing FLAG-tagged hTERT. At 20 h after transfection, a total cell lysate was prepared and subjected to immunoprecipitation (IP) without antibody or using anti-GST, anti-FLAG, anti-Sm (Y12), or anti-TEP1. Immunoprecipitates were analyzed by SDS-PAGE and Western blotting for endogenous SMN. The lysate lane corresponds to 5% of the total cell lysate used for the immunoprecipitation. (C) Nucleolar-enriched nuclear extracts were prepared from HeLa cells and subjected to immunoprecipitation (IP) using anti-GST, anti-Sm (Y12), and two different affinity-purified hTERT antibodies. Immunoprecipitates were analyzed by SDS-PAGE and Western blotting for endogenous hTERT (top) and SMN (bottom). Five percent of the cytosolic (C) and nucleolar-enriched nuclear (N) extracts were also loaded.
Figure 2
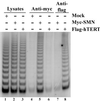
SMN associates with catalytically active telomerase. 293 cells were transiently transfected with DNA constructs expressing Myc-tagged SMN (lanes 2, 5, and 7), FLAG-tagged hTERT (lanes 3, 6, and 8), or with vector alone (mock; lanes 1 and 4). Total cell lysates were prepared and subjected to immunoprecipitation using either anti-Myc (lanes 4–6) or anti-FLAG (lanes 7 and 8). Immunoprecipitates were analyzed for telomerase activity by the TRAP assay. Of the total cell lysates (lanes 1–3), 0.5% were also assayed for telomerase activity.
Figure 3
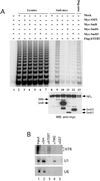
The hTR and telomerase activity are not associated with the Sm protein complex. (A) 293 cells were transiently transfected with DNA constructs expressing Myc-SMN (lanes 3 and 9), Myc-SmB (lanes 4 and 10), Myc-SmD1 (lanes 5 and 11), Myc-SmD3 (lanes 6 and 12), FLAG-hTERT (lanes 7 and 13), or with vector alone (mock; lanes 2 and 8). At 20 h after transfection, total cell lysates were prepared and subjected to immunoprecipitation using either anti-Myc (lanes 8–12) or anti-FLAG (lane 13). Immunoprecipitates were analyzed for telomerase activity by the TRAP assay (top) and for protein content by Western blotting (WB) using anti-myc (bottom). Of the total cell lysates (lanes 2–7) or lysis buffer (lane 1), 1% were analyzed for telomerase activity. (B) A total cell lysate from HeLa cells was prepared and subjected to immunoprecipitation using anti-Sm (Y12-lane 2), affinity-purified anti-hTERT (lane 3), anti-TMG (lane 4), or anti-GST (lane 5). Immunoprecipitates were analyzed by denaturing PAGE and Northern blotting for endogenous hTR (top), U1 snRNA (middle), and U6 snRNA (bottom). The input lane (lane 1) was loaded with 2.5% of the total RNA extracted from the HeLa total cell lysate. For hTR, exposure time was three times longer than for U1 and U6.
Figure 4
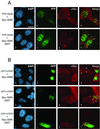
The expression of a dominant-negative mutant of SMN (SMNΔN27) perturbs the nuclear localization of hTERT. (A) HeLa cells were transiently cotransfected with DNA constructs expressing either Myc-SMN and YFP-SmB (a–d) or Myc-SMNΔN27 and YFP-SmB (e–h). The fixed and permeabilized cells were stained for Myc-SMN (c) and Myc-SMNΔN27 (g) using anti-Myc. DNA stained with DAPI shows the nucleus of each cell (a and e). Images b and c, and f and g, are merged to form d and h, respectively. (B) HeLa cells were transiently cotransfected with DNA constructs expressing either Myc-SMN and GFP-hTERT (a–d) or Myc-SMNΔN27 and GFP-hTERT (e–l). The fixed and permeabilized cells were stained for Myc-SMN (c) and Myc-SMNΔN27 (g and k) using anti-Myc. DNA stained with DAPI shows the nucleus of each cell (a, e, and i). Images b and c, f and g, and j and k are merged to form d, h, and l, respectively. The arrows point to the nuclear gems and the arrowheads to the SMNΔN27-induced cytoplasmic accumulations. Bar, 12 μm.
Figure 5
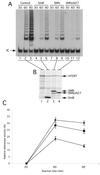
Expression of SMNΔN27 decreases human telomerase activity reconstitution in RRLs. (A) hTERT was synthesized in RRL in the presence of hTR, [35S]methionine, and equal amounts of DNA constructs expressing Myc-SmB (lanes 4–6), Myc-SMN (lanes 7–9), Myc-SMNΔN27 (lanes 10–12), or no additional DNA (lanes 1–3). At 30, 60, and 90 min after the start of the RRL reactions, telomerase activity was assayed by the TRAP assay. Each TRAP reaction included an internal control (IC) to normalize for variation in PCR efficiency. (B) At 90 min after the start of the RRL reactions, equal amounts were analyzed by SDS-PAGE and autoradiography. (C) The telomerase activity was calculated as the ratio between the intensity of the telomerase ladder products and the intensity of the internal PCR control. A ratio of this telomerase activity value to the amount of in vitro–translated hTERT measured by the intensity of the S35-labeled hTERT was calculated to generate the relative telomerase activity. The activities from three independent experiments performed in the presence of SmB (diamonds), wild-type SMN (squares), and SMNΔN27 (triangles) were averaged and compared relative to RRL reactions in which no additional DNA construct was included.
Similar articles
- Gemin8 is a novel component of the survival motor neuron complex and functions in small nuclear ribonucleoprotein assembly.
Carissimi C, Saieva L, Baccon J, Chiarella P, Maiolica A, Sawyer A, Rappsilber J, Pellizzoni L. Carissimi C, et al. J Biol Chem. 2006 Mar 24;281(12):8126-34. doi: 10.1074/jbc.M512243200. Epub 2006 Jan 24. J Biol Chem. 2006. PMID: 16434402 - Protein phosphatase 4 interacts with the Survival of Motor Neurons complex and enhances the temporal localisation of snRNPs.
Carnegie GK, Sleeman JE, Morrice N, Hastie CJ, Peggie MW, Philp A, Lamond AI, Cohen PT. Carnegie GK, et al. J Cell Sci. 2003 May 15;116(Pt 10):1905-13. doi: 10.1242/jcs.00409. Epub 2003 Mar 18. J Cell Sci. 2003. PMID: 12668731 - Gemin5, a novel WD repeat protein component of the SMN complex that binds Sm proteins.
Gubitz AK, Mourelatos Z, Abel L, Rappsilber J, Mann M, Dreyfuss G. Gubitz AK, et al. J Biol Chem. 2002 Feb 15;277(7):5631-6. doi: 10.1074/jbc.M109448200. Epub 2001 Nov 19. J Biol Chem. 2002. PMID: 11714716 - The SMN complex.
Gubitz AK, Feng W, Dreyfuss G. Gubitz AK, et al. Exp Cell Res. 2004 May 15;296(1):51-6. doi: 10.1016/j.yexcr.2004.03.022. Exp Cell Res. 2004. PMID: 15120993 Review. - The SMN complex: an assembly machine for RNPs.
Battle DJ, Kasim M, Yong J, Lotti F, Lau CK, Mouaikel J, Zhang Z, Han K, Wan L, Dreyfuss G. Battle DJ, et al. Cold Spring Harb Symp Quant Biol. 2006;71:313-20. doi: 10.1101/sqb.2006.71.001. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381311 Review.
Cited by
- Identification of processing elements and interactors implicate SMN, coilin and the pseudogene-encoded coilp1 in telomerase and box C/D scaRNP biogenesis.
Poole AR, Enwerem II, Vicino IA, Coole JB, Smith SV, Hebert MD. Poole AR, et al. RNA Biol. 2016 Oct 2;13(10):955-972. doi: 10.1080/15476286.2016.1211224. Epub 2016 Jul 15. RNA Biol. 2016. PMID: 27419845 Free PMC article. - Depletion of SMN by RNA interference in HeLa cells induces defects in Cajal body formation.
Girard C, Neel H, Bertrand E, Bordonné R. Girard C, et al. Nucleic Acids Res. 2006 May 31;34(10):2925-32. doi: 10.1093/nar/gkl374. Print 2006. Nucleic Acids Res. 2006. PMID: 16738131 Free PMC article. - Maturation of small nucleolar RNAs: from production to function.
Webster SF, Ghalei H. Webster SF, et al. RNA Biol. 2023 Jan;20(1):715-736. doi: 10.1080/15476286.2023.2254540. Epub 2023 Oct 5. RNA Biol. 2023. PMID: 37796118 Free PMC article. Review. - Human telomerase activity regulation.
Wojtyla A, Gladych M, Rubis B. Wojtyla A, et al. Mol Biol Rep. 2011 Jun;38(5):3339-49. doi: 10.1007/s11033-010-0439-x. Epub 2010 Nov 18. Mol Biol Rep. 2011. PMID: 21086176 Free PMC article. Review. - SMN and coilin negatively regulate dyskerin association with telomerase RNA.
Poole AR, Hebert MD. Poole AR, et al. Biol Open. 2016 Jun 15;5(6):726-35. doi: 10.1242/bio.018804. Biol Open. 2016. PMID: 27215323 Free PMC article.
References
- Bachand F, Autexier C. Functional reconstitution of human telomerase expressed in Saccharomyces cerevisiae. J Biol Chem, 1999;274:38027–38031. - PubMed
- Beattie TL, Zhou W, Robinson MO, Harrington L. Reconstitution of human telomerase activity in vitro. Curr Biol, 1998;8:177–180. - PubMed
- Chen J-L, Blasco MA, Greider CW. Secondary structure of vertebrate telomerase RNA. Cell, 2000;100:503–514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases