An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome - PubMed (original) (raw)
An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome
Deborah U Frank et al. Development. 2002 Oct.
Abstract
Deletion of chromosome 22q11, the most common microdeletion detected in humans, is associated with a life-threatening array of birth defects. Although 90% of affected individuals share the same three megabase deletion, their phenotype is highly variable and includes craniofacial and cardiovascular anomalies, hypoplasia or aplasia of the thymus with associated deficiency of T cells, hypocalcemia with hypoplasia or aplasia of the parathyroids, and a variety of central nervous system abnormalities. Because ablation of neural crest in chicks produces many features of the deletion 22q11 syndrome, it has been proposed that haploinsufficiency in this region impacts neural crest function during cardiac and pharyngeal arch development. Few factors required for migration, survival, proliferation and subsequent differentiation of pharyngeal arch neural crest and mesoderm-derived mesenchyme into their respective cardiovascular, musculoskeletal, and glandular derivatives have been identified. However, the importance of epithelial-mesenchymal interactions and pharyngeal endoderm function is becoming increasingly clear. Fibroblast growth factor 8 is a signaling molecule expressed in the ectoderm and endoderm of the developing pharyngeal arches and known to play an important role in survival and patterning of first arch tissues. We demonstrate a dosage-sensitive requirement for FGF8 during development of pharyngeal arch, pharyngeal pouch and neural crest-derived tissues. We show that FGF8 deficient embryos have lethal malformations of the cardiac outflow tract, great vessels and heart due, at least in part, to failure to form the fourth pharyngeal arch arteries, altered expression of Fgf10 in the pharyngeal mesenchyme, and abnormal apoptosis in pharyngeal and cardiac neural crest. The Fgf8 mutants described herein display the complete array of cardiovascular, glandular and craniofacial phenotypes seen in human deletion 22q11 syndromes. This represents the first single gene disruption outside the typically deleted region of human chromosome 22 to fully recapitulate the deletion 22q11 phenotype. FGF8 may operate directly in molecular pathways affected by deletions in 22q11 or function in parallel pathways required for normal development of pharyngeal arch and neural crest-derived tissues. In either case, Fgf8 may function as a modifier of the 22q11 deletion and contribute to the phenotypic variability of this syndrome.
Figures
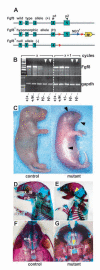
Fig. 1
The presence of the neor gene in the 3′untranslated region (UTR) of the Fgf8 gene has transcriptional and morphological consequences. (A) The _Fgf8_H hypomorphic and Fgf8- alleles. The neomycin phosphotransferase gene (neor, blue arrow) and alkaline phosphatase reporter gene (AP, yellow block arrowhead) are located in the 3′UTR of Fgf8. PCR primers used to amplify exon 5 containing reverse transcription (RT) products are shown as black arrowheads (P1 and P2). LoxP sites are shown as red arrowheads. The null allele lacks Fgf8 exon 5 as previously described (Moon and Capecchi, 2000). If recombined by Cre recombinase, the _Fgf8_H allele produces the AP reporter regulated by the Fgf8 promoter. (B) Semi-quantitative RT/PCR of cDNAs prepared by reverse transcription of mRNA isolated from E9.5 embryos. The amount of exon 5 containing Fgf8 mRNA is decreased in Fgf8 mutants (_Fgf8_H/-, white arrowheads) when compared with wild type (Fgf8+/+), null heterozygote (Fgf8+/-) or hypomorph allele heterozygote (_Fgf8_H/+) littermate controls. The results displayed show the amplification products obtained with amplification cycles of X or X+1 to assure that the amplification was performed within the linear range for each product and that the signal obtained was not saturated. The control panel shows RT-PCR products using GAPDH primers and confirms that specimens from the _Fgf8_H/- mutant embryos contain comparable amounts of total mRNA with controls, although the amount of Fgf8 mRNA in _Fgf8_H/- compound heterozygous, hypomorphic mutants is markedly decreased. (C) Newborn _Fgf8_H/- mutant and _Fgf8_H/+ control. The mutant is smaller, cyanotic and edematous with obvious craniofacial abnormalities (arrowheads). All of the mutants had CNS and mandibular malformations, 30% had a cleft palate. (D-G) Skeleton preps of _Fgf8_H/- mutant and control. (E) The mutant exhibits micrognathia, fusion of the maxilla (mx), jugal bone (jg) and mandible (mb), and absence of the tympanic ring (t, yellow arrowhead). (G) Cleft palate (arrowhead) in a mutant resulting from failure of fusion of the palatine shelves (pl) with the maxillary shelves (ms).
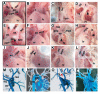
Fig. 2
E18.5 _Fgf8_H/- mutants exhibit lethal cardiovascular malformations. (A,E) Frontal and left oblique views of wild-type newborn heart and great vessels: the aorta (ao) arises from the left ventricle (lv) and is located to the right and posterior to the main pulmonary artery (mpa); the left aortic arch (aa) gives rise to the brachiocephalic (b), left common carotid (lcc) and left subclavian (lsc) and the aorta descends on the left (dao). The MPA arises from the right ventricle (rv). The ductus arteriosus (da) is closing (E, arrowhead). (B-D) Persistent Truncus Arteriosus results from failure of outflow tract septation.(B) Frontal view; a single vessel, the truncus (t), originates from the right ventricle (RV). (C) Left lateral view reveals origin of the left branch pulmonary artery (pa) and LCC from the truncal vessel. A right aortic arch was noted (raa). Broken lines have been superimposed to help clarify the anatomy. (D) Viewed from inside the RV, the truncal vessel overrides a large membranous ventricular septal defect (VSD, arrowhead), which is the only path of egress from the LV. The right atrium (ra) has been removed. (F-H) Double outlet right ventricle results from failure of outflow tract rotation and alignment. (F) Frontal view, MPA is anterior and towards the left of the aorta (Ao). (G) Right lateral view after removal of the right atrium and RV-free wall; note origins of Ao and MPA from the RV, there is a subaortic VSD (arrowhead), a right aortic arch and right patent ductus arteriosus (pda). (H) Left lateral view after removal of the LV free wall shows a probe passing from left ventricle through VSD into Ao; an atrial septal defect is visible from the left atrium (la, arrowhead). (I-L) D-Transposition of the great arteries results from failure of outflow tract rotation and alignment. (I) Frontal view; parallel circulation due to aberrant origin of the Ao anteriorly and from the RV and the MPA posteriorly from the LV. (J) Right lateral view shows right aortic arch (arrowhead), right descending aorta (dao) and right atrium (ra). (K) Right oblique view with probe passing from RV into Ao. (L) Left lateral view with probe passing from LV into MPA; there was no VSD. (M-P) Corrosion casts of the embryonic arterial vasculature reveal aortic arch anomalies in _Fgf8_H/- mutants. (M) Dorsal view of a control embryo; note continuity between ascending and descending aorta via the left aortic arch (laa), which is derived from the embryonic left fourth aortic arch artery. (N) Right dorsal oblique view of a control embryo; the PDA is visible, as is the LAA. (O) Posterior view of a mutant with interrupted aortic arch, type B; note discontinuity between ascending and descending aortas (red arrowhead) with the carotids arising from the ascending aorta and the left subclavian and descending aorta arising from the patent ductus arteriosus. (P) Dorsal oblique view of a mutant with a right aortic arch. A left-sided PDA and right aortic arch (raa) are shown. Broken lines have been superimposed to clarify the anatomy. This is a vascular ring encompassing the trachea and esophagus, which can cause airway obstruction and swallowing difficulties.
Fig. 3
Formation of the fourth pharyngeal arch arteries (PAAs) is abnormal in _Fgf8_H/- mutants. (A-F) Left lateral view after intracardiac ink injection to visualize the developing PAAs at E9.5 (A,B), 10.5 (C,D) and 11.5 (E,F) in Fgf8 mutants (B,D,F) and somite/stage matched controls (A,C,E). The PAAs are numbered and the aortic sac (as) and dorsal aorta (da) labeled. Note complete absence of the fourth PAAs in the mutants at E10.5 and E11.5, although the third and sixth PAAs are present. (G-J) Formation of the third pharyngeal pouch and fourth pharyngeal arch is disrupted in _Fgf8_H/- mutants. Immunohistochemistry for PECAM to stain the endothelium of the PAAs; plane of transverse sections through the arches is shown in inset of H. (G,I) control embryo, ventral (G) and dorsal (I); the ventral section is at the junction of the aortic sac with the origins of the second, third and fourth arch arteries. (H,J) Mutant embryo, ventral (H) and dorsal (J) transverse sections; the fourth pharyngeal arch tissue is hypoplastic, fused to the third arch and avascular on the left, although disorganized PECAM-positive endothelial cells are present (arrowheads); there is no third pouch on the left. The right fourth PAA is minimally endothelialized and never connects to the dorsal aorta (data not shown). The sixth arch arteries are larger than normal and have already fused with the dorsal aorta in section J. The rostral pharynx (rp) and caudal pharynx (cp) are labeled and the pharyngeal arches are numbered and labeled left (l) or right (r).
Fig. 4
Mesenchymal expression of Fgf10 is decreased, while Mfh1, Hrt1 and Tbx1 appear normal in _Fgf8_H/- mutants. Views of the pharyngeal arch area, head is at the top. Arches are numbered in the controls. (A) Mfh1, (B) Hrt1, (C) Tbx1 and (D,E) Fgf10 expression in E9.5 and E10.5 control and mutant embryos. (E) Note the markedly decreased signal in the pharyngeal arches, including area of the developing fourth arch and putative secondary heart field, although expression in limb bud and ototcyst is relatively intact.
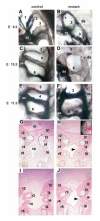
Fig. 5
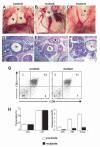
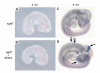
_Fgf8_H/- mutants display glandular phenotypes and T-cell deficiency attributable to altered pharyngeal pouch development. eighty percent of mutants had thymic (Ty) hypo/aplasia and 50% had parathyroid hypo/aplasia. (A-C) Thoracotomies, (D-F) Hematoxylin and Eosin stained paraffin wax-embedded sections. The trachea (tr), esophagus (e) and thyroids (td) are also indicated. (A) Normal bi-lobed thymus in a control E18.5 embryo. (B) Hypoplastic, mono-lobed thymus in a mutant. (C) Thymic aplasia in a mutant (arrowhead). (D) Normal bilateral parathyroid glands in a control E18.5 embryo (arrowheads). (E) Parathyroid aplasia in a mutant (arrowheads), the entire cervical region of this animal was sectioned and no parathyroids were detected. (F) Ectopic, hypoplastic parathyroid (arrowhead) adjacent to internal carotid vessel (v) in a mutant. (G) Representative FACS plots of control and mutant thymi. (H) Percent (left panel) and total (right panel) CD4-CD8-double negative (DN) and CD4+CD8+double positive (DP) thymocytes in _n_=9 control embryos or _n_=2 mutant embryos with thymic hypoplasia. Mutants with thymic hypoplasia have 20% of the normal number of T cells. Note that 50% of mutant embryos have no thymus. Data are shown as mean ± s.e.m.
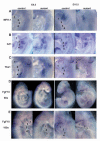
Fig. 6
Premigratory and migratory cardiac and pharyngeal neural crest do not express _Fgf8. Wnt1_-Cre was used to drive expression of the alkaline phosphatase (AP) reporter gene from the _Fgf8_H allele in premigratory and migrating neural crest; Cre-mediated recombination of the _Fgf8_H allele results in expression of the AP gene regulated by the Fgf8 promoter and is detected as blue staining. (A) E8.5 control embryo bearing the _Fgf8_H allele. (B) E8.5 embryo bearing the _Fgf8_H and the _Wnt1_-Cre transgene; no staining is detected, indicating that Fgf8 is not expressed in any cells of the Wnt1-Cre lineage at this stage. (C) E9.5 control embryo with the _Fgf8_H allele. (D) E9.5 embryo bearing the _Fgf8_H allele and the Wnt1-Cre transgene; blue staining reveals overlapping domains of Wnt1 and Fgf8 expression in the isthmus and frontonasal prominences (arrows). No activity in neural crest of rhombomeres 6-8 (R6, R7 arrowheads), which are the origin of premigratory third and fourth pharyngeal and cardiac neural crest.
Fig. 7
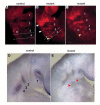
There is increased apoptosis in the pharyngeal arches of _Fgf8_H/- mutants in domains adjacent and remote to Fgf8 expression. Whole mount TUNEL analysis was performed on 25 somite, stage-matched control. (A) and mutant (B,C) embryos. Pharyngeal arches are numbered and the otocyst is labeled (O); note the increased number of apoptotic cells in normal domains in the rostral arches (white arrowheads in B) and abnormal domains of apoptosis in the postotic region of the developing fourth and sixth arches (yellow arrowheads) in the mutants. Fgf8 expression by in situ hybridization on E9.5 control and mutant embryos. (D,E) Pharyngeal region of control embryo and (E) mutant embryo; note severely decreased quantity of Fgf8 message in the mutant (red arrowheads, consistent with results in Fig. 1B). Domains of apoptosis in B and C are seen both adjacent, and relatively distant, to normal regions of Fgf8 expression.
Fig. 8
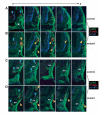
Migrating cardiac neural crest undergoes abnormal apoptosis in _Fgf8_H/- mutants. Fluorescent immunohistochemistry was performed on transverse cryosections using a combination of anti-AP-2/FITC (green), TUNEL/Texas red (red) and either (A,B) anti-Tuj1/Cy5 (blue) which labels neuronal tubulin, or (C,D) antiPHH3/Cy5 (blue), which labels chromatin in proliferating cells. (A,B) Twenty-five somite stage matched (A) control and (B) mutant; plane and region of sections are illustrated by broken lines in Fig. 7B. White asterisks mark the third PAA in parallel. (C,D) Twenty-nine somite stage matched (C) control and (D) mutant; sections begin just posterior to the junction of the third aortic arch artery with the dorsal aorta (ao). From left to right, sections proceed from anterior to posterior through the post-otic region. Blue arrowheads indicate normal domains of apoptosis, including regions of developing ganglia and neural tube that label with anti-Tuj1. Yellow arrowheads indicate increased numbers of apoptotic cells in normal domains or abnormal domains of apoptosis in mutant neural crest mesenchyme. Yellow staining indicates double labeling of cardiac/pharyngeal neural crest cells with anti-AP-2α and TUNEL, and reveals abnormal apoptosis in lateral neural crest of the developing fourth arch. No reproducible differences in PHH3 staining were detected at this stage.
Similar articles
- Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse.
Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Abu-Issa R, et al. Development. 2002 Oct;129(19):4613-25. doi: 10.1242/dev.129.19.4613. Development. 2002. PMID: 12223417 - Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development.
Macatee TL, Hammond BP, Arenkiel BR, Francis L, Frank DU, Moon AM. Macatee TL, et al. Development. 2003 Dec;130(25):6361-74. doi: 10.1242/dev.00850. Development. 2003. PMID: 14623825 Free PMC article. - Foxi transcription factors promote pharyngeal arch development by regulating formation of FGF signaling centers.
Edlund RK, Ohyama T, Kantarci H, Riley BB, Groves AK. Edlund RK, et al. Dev Biol. 2014 Jun 1;390(1):1-13. doi: 10.1016/j.ydbio.2014.03.004. Epub 2014 Mar 18. Dev Biol. 2014. PMID: 24650709 Free PMC article. - Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.
Stalmans I. Stalmans I. Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review. - Neural Crest.
Thattaliyath BD, Firulli AB. Thattaliyath BD, et al. Adv Exp Med Biol. 2024;1441:125-143. doi: 10.1007/978-3-031-44087-8_6. Adv Exp Med Biol. 2024. PMID: 38884708 Review.
Cited by
- Thymic epithelial cell development and differentiation: cellular and molecular regulation.
Sun L, Luo H, Li H, Zhao Y. Sun L, et al. Protein Cell. 2013 May;4(5):342-55. doi: 10.1007/s13238-013-3014-0. Epub 2013 Apr 15. Protein Cell. 2013. PMID: 23589020 Free PMC article. Review. - Two developmentally distinct populations of neural crest cells contribute to the zebrafish heart.
Cavanaugh AM, Huang J, Chen JN. Cavanaugh AM, et al. Dev Biol. 2015 Aug 15;404(2):103-12. doi: 10.1016/j.ydbio.2015.06.002. Epub 2015 Jun 15. Dev Biol. 2015. PMID: 26086691 Free PMC article. - 22q11.2 Deletion Syndrome: Laboratory Diagnosis and TBX1 and FGF8 Mutation Screening.
Sgardioli IC, Vieira TP, Simioni M, Monteiro FP, Gil-da-Silva-Lopes VL. Sgardioli IC, et al. J Pediatr Genet. 2015 Mar;4(1):17-22. doi: 10.1055/s-0035-1554976. J Pediatr Genet. 2015. PMID: 27617111 Free PMC article. - Analyses of the associations between the genes of 22q11 deletion syndrome and schizophrenia.
Arinami T. Arinami T. J Hum Genet. 2006;51(12):1037-1045. doi: 10.1007/s10038-006-0058-5. Epub 2006 Sep 13. J Hum Genet. 2006. PMID: 16969581 Review. - Three phases of DiGeorge/22q11 deletion syndrome pathogenesis during brain development: patterning, proliferation, and mitochondrial functions of 22q11 genes.
Meechan DW, Maynard TM, Tucker ES, LaMantia AS. Meechan DW, et al. Int J Dev Neurosci. 2011 May;29(3):283-94. doi: 10.1016/j.ijdevneu.2010.08.005. Epub 2010 Sep 15. Int J Dev Neurosci. 2011. PMID: 20833244 Free PMC article. Review.
References
- Bamforth SD, Braganca J, Eloranta JJ, Murdoch JN, Marques FI, Kranc KR, Farza H, Henderson DJ, Hurst HC, Bhattacharya S. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat Genet. 2001;29:469–474. - PubMed
- Birgbauer E, Sechrist J, Bronner-Fraser M, Fraser S. Rhombomeric origin and rostrocaudal reassortment of neural crest cells revealed by intravital microscopy. Development. 1995;121:935–945. - PubMed
- Bockman DE, Kirby ML. Dependence of thymus development on derivatives of the neural crest. Science. 1984;223:498–500. - PubMed
- Bockman DE, Redmond ME, Kirby ML. Alteration of early vascular development after ablation of cranial neural crest. Anat Rec. 1989;225:209–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases