The cyclin-dependent kinase 5 activators p35 and p39 interact with the alpha-subunit of Ca2+/calmodulin-dependent protein kinase II and alpha-actinin-1 in a calcium-dependent manner - PubMed (original) (raw)
The cyclin-dependent kinase 5 activators p35 and p39 interact with the alpha-subunit of Ca2+/calmodulin-dependent protein kinase II and alpha-actinin-1 in a calcium-dependent manner
Rani Dhavan et al. J Neurosci. 2002.
Abstract
Cyclin-dependent kinase 5 (Cdk5) is a critical regulator of neuronal migration in the developing CNS, and recent studies have revealed a role for Cdk5 in synaptogenesis and regulation of synaptic transmission. Deregulation of Cdk5 has been linked to the pathology of neurodegenerative diseases such as Alzheimer's disease. Activation of Cdk5 requires its association with a regulatory subunit, and two Cdk5 activators, p35 and p39, have been identified. To gain further insight into the functions of Cdk5, we identified proteins that interact with p39 in a yeast two-hybrid screen. In this study we report that alpha-actinin-1 and the alpha-subunit of Ca2+/calmodulin-dependent protein kinase II (CaMKIIalpha), two proteins localized at the postsynaptic density, interact with Cdk5 via their association with p35 and p39. CaMKIIalpha and alpha-actinin-1 bind to distinct regions of p35 and p39 and also can interact with each other. The association of CaMKIIalpha and alpha-actinin-1 to the Cdk5 activators, as well as to each other, is stimulated by calcium. Further, the activation of glutamate receptors increases the association of p35 and p39 with CaMKIIalpha, and the inhibition of CaMKII activation diminishes this effect. The glutamate-mediated increase in association of p35 and CaMKIIalpha is mediated in large part by NMDA receptors, suggesting that cross talk between the Cdk5 and CaMKII signal transduction pathways may be a component of the complex molecular mechanisms contributing to synaptic plasticity, memory, and learning.
Figures
Fig. 1.
CaMKIIα and α-actinin-1 interact with the Cdk5 activators p39 and p35, and with each other, in a yeast two-hybrid system. A, A schematic of full-length CaMKIIα and α-actinin-1 proteins is presented above a diagram of the protein encoded by the cDNA clones identified in the screen. The_numbers_ in parentheses indicate the number of copies of CaMKIIα and α-actinin-1 cDNAs isolated in the screen. CaMKIIα contains an N-terminal kinase domain, a Ca2+/calmodulin binding domain (CBD), and an association domain (AD). Both CaMKIIα cDNAs identified in the screen contain the entire open reading frame (amino acid residues 1–478). α-Actinin-1 is composed of an N-terminal actin binding domain, a central region containing four spectrin repeats (I–IV), and a C terminus with two EF-hand motifs. The cDNA identified in the screen encodes amino acid residues 363–892 of α-actinin-1. B, cDNAs encoding full-length p39 (residues 1–369) and p35 (residues 1–307) or N- and C-terminal truncation mutants of p39 and p35 were tested for association with the CaMKIIα and α-actinin-1 clones identified in the yeast two-hybrid screen. Strength of an interaction is indicated by ++++, +++, ++, or + on the basis of β-galactosidase assays, whereas – denotes no interaction (see Materials and Methods). C, Full-length or truncation mutants of CaMKIIα were tested for their ability to interact with full-length p35 and p39 in a yeast two-hybrid β-galactosidase assay. A schematic of the proteins encoded by the CaMKIIα cDNAs is indicated. D, Full-length or truncation mutants of α-actinin-1 were tested for their interaction with p35 and p39. A schematic representation of the proteins encoded by α-actinin-1 cDNAs is presented. The N terminus contains an actin binding domain (ABD); the central region contains four spectrin-like repeats (SRI–IV). The C terminus contains a pair of EF-hand motifs (EF-H) depicted by two vertical lines. E, Full-length or truncation mutants of CaMKII were tested for their ability to interact with full-length α-actinin-1 in a yeast two-hybrid assay.F, Full-length or truncation mutants of α-actinin-1 were tested for their ability to interact with full-length CaMKIIα in the yeast two-hybrid assay.
Fig. 2.
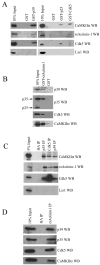
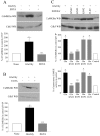
CaMKIIα, α-actinin-1, and Cdk5 and its activators interact in adult rat brain. A, GST fusion proteins of p39 (GST-p39), p25 (GST-p25), Cdk5 (GST-Cdk5), or GST alone were incubated with adult rat brain lysate. Then 10% of the lysates used for the pulldowns (10% Input) and the associated proteins were resolved by SDS-PAGE and detected by immunoblotting with CaMKIIα, α-actinin-1, Cdk5, and Lis1 antibodies, as indicated on the_right_. B, GST alone and a GST fusion protein encoding residues 363–892 of actinin-1 (GST-α-actinin-1) were incubated with adult rat brain lysate, and the associated proteins were detected by Western blotting with p39, p35, Cdk5, and CaMKIIα antibodies, as indicated on the right. The p35 antibody recognizes both full-length p35 and the N-terminal truncation product termed p25, as indicated by the arrows on the left.C, CaMKIIα and α-actinin-1 coimmunoprecipitate with Cdk5 and its activators. p35, p39, Cdk5, and HA antibodies (indicated at the top of the lanes) were used for immunoprecipitations from adult rat brain lysate. Then 5% of the lysate used for the immunoprecipitation (5% Input) and the immunoprecipitated proteins were resolved by SDS-PAGE and detected by immunoblotting with the antibodies indicated on the_right_. D, α-Actinin-1 or HA antibody was used for immunoprecipitations from adult rat brain lysate. Then 10% of the proteins used for the immunoprecipitations (10% Input) and the associated proteins were resolved by SDS-PAGE and detected by immunoblotting with the antibodies indicated on the_right_.
Fig. 3.
CaMKIIα, α-actinin-1, and Cdk5 and its activators colocalize in hippocampal neurons. Hippocampal neurons maintained in culture for 3 weeks were immunostained as described in Materials and Methods. A–L, Images were acquired by using a 60× oil-immersion objective. Scale bar in A, 10 μ
m
(also applies to A–C,E–G, and I–K).M–P, Image was acquired by using a 100× oil-immersion objective. Scale bar in M, 10 μ
m
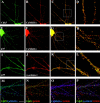
(also applies to N–P). A–D, Colocalization of Cdk5 and CaMKIIα. Hippocampal neurons were double stained for Cdk5 (A, green) and CaMKII (B,red). C, The images shown in_A_ and B are combined to reveal the colocalization of Cdk5 (green) and CaMKII (red) in yellow. The boxed area in C is presented in D at 3.5-fold magnification. E–H, Colocalization of p35 and CaMKIIα. Hippocampal neurons were double stained for p35 (E, green) and CaMKIIα (F, red). G, The images were merged to indicate colocalization between p35 (green) and CaMKIIα (red) in_yellow_. The boxed region of the image in_G_ is presented at 3.5-fold magnification in_H_. I–L, Colocalization of p35 and α-actinin-1. Hippocampal neurons were double stained for p35 (I, green) and α-actinin-1 (J, red). L, The images were merged to indicate colocalization of p35 (green) and α-actinin-1 (red) in_yellow_. The boxed region of the image in_K_ is presented at 3.5-fold magnification in_L_. M–P, Triple colocalization of Cdk5, CaMKIIα, and α-actinin-1. IgG- and IgM-specific secondary antibodies were used to triple-stain hippocampal neurons with Cdk5, CaMKIIα, and α-actinin-1 antibodies (see Materials and Methods).M, Colocalization of Cdk5 (green) and CaMKII (blue) in triple-stained neurons is revealed by the blue-green color. N, Colocalization of Cdk5 (green) and α-actinin-1 (red) in triple-stained hippocampal neurons is visible as yellow. O, Colocalization of CaMKIIα (blue) and α-actinin-1 (red) in triple-stained hippocampal neurons is visible as pink.P, Triple colocalization of Cdk5 (green), α-actinin-1 (red), and CaMKIIα (blue) in triple-stained neurons is visible as_white_.
Fig. 4.
Ca2+-dependent association of CaMKIIα, α-actinin-1, and the Cdk5 activators. A, Ca2+ enhances the coimmunoprecipitation of CaMKIIα and α-actinin-1 with p35. EDTA (1 m
m
) or CaCl2 was added to adult rat brain lysate to a final concentration indicated above the lanes (0–5 m
m
). p35 antibody (p35 IP) or no antibody (no Ab) was used for immunoprecipitations from these lysates, and the immunoprecipitated samples were resolved by SDS-PAGE. The amounts of associated α-actinin-1 (top), CaMKIIα (middle), and Cdk5 (bottom) were analyzed by Western blots. Then 5% of the lysates used for the immunoprecipitations (Lysate) was analyzed by Western blotting for the levels of p35, α-actinin-1, CaMKIIα, and Cdk5. B, Ca2+ enhances the coimmunoprecipitation of CaMKIIα and α-actinin-1 with p39. EGTA (1 m
m
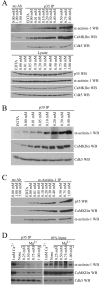
) or CaCl2 was added to adult rat brain lysate to a final concentration indicated above the lanes. p39 antibody (p39 IP) was used for immunoprecipitations from these lysates; the amounts of associated α-actinin-1 (top), CaMKIIα (middle), and Cdk5 (bottom) were analyzed by Western blots.C, Ca2+ enhances the coimmunoprecipitation of p35 and CaMKIIα with α-actinin-1. The same samples described in A were used for immunoprecipitations with the α-actinin-1 antibody (α-actinin-1 IP) or no antibody (no Ab). The amounts of associated p35 (top), CaMKIIα (middle), and α-actinin-1 (bottom) were analyzed by Western blots.D, Magnesium does not enhance the coimmunoprecipitation of CaMKIIα and α-actinin-1 with p35. CaCl2(Ca 2+) or MgCl2(Mg2+) was added to adult rat brain lysate to a final concentration indicated above the _lanes._p35 antibody was used for immunoprecipitations from these lysates. Then 10% of the lysates used for the immunoprecipitations (10% Input) and the immunoprecipitated samples (p35 IP) were resolved by SDS-PAGE. The amounts of associated α-actinin-1 (top), CaMKIIα (middle), and Cdk5 (bottom) were analyzed by Western blots.
Fig. 5.
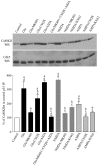
Glutamate treatment of dissociated hippocampal neurons enhances the association of CaMKIIα and p35.A, Dissociated hippocampal neurons in culture for 2 weeks were treated (Glu/Gly) with buffer (−) or 100 μ
m
glutamate/10 μ
m
glycine (+) for 5 min. Crude synaptosome fractions prepared from these neurons were used for the immunoprecipitation of p35. Then 10% of the proteins used for the immunoprecipitations (10% Input) and the p35-associated proteins (p35 IP) were resolved by SDS-PAGE. The amounts of CaMKIIα (top), α-actinin-1 (middle), and Cdk5 (bottom) were analyzed by immunoblotting. Quantitative analysis from three independent experiments is presented in the histogram. The amount of CaMKIIα, α-actinin-1, and Cdk5 immunoprecipitated with p35 is presented as a percentage of the values that were measured in control treatments and is the mean ± SEM of three independent experiments. *Statistically different (p < 0.05) from control values by Student's t test. B, The same lysates described in A were used for immunoprecipitation with α-actinin-1 antibody. Then 10% of the proteins used for the immunoprecipitations (10% Input) and the α-actinin-1-associated proteins (α-actinin-1 IP) were resolved by SDS-PAGE; the amounts of p35 (top) and CaMKIIα (bottom) were analyzed by immunoblotting. Quantitative analysis from three independent experiments is presented in the histogram. The amount of CaMKIIα and p35 immunoprecipitated with α-actinin-1 is presented as a percentage of the values that were measured in control treatments and is the mean ± SEM of three independent experiments.C, Cdk5 kinase activity is unaltered by glutamate treatment of hippocampal neurons. Dissociated hippocampal neurons were treated with either buffer (Control) or 100 μ
m
glutamate/10 μ
m
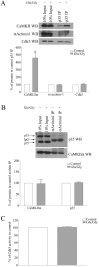
glycine (Glu/Gly), as described in A. The Cdk5/p35 complex was immunoprecipitated with p35 antibody, and Cdk5 kinase activity was measured in an in vitro kinase assay toward Histone H1. The Cdk5 kinase activity is presented as a percentage of the values that were measured from control-treated neurons and is the mean ± SEM from three independent experiments.
Fig. 6.
Ca2+ influx and CaMKII activity are required for the glutamate-mediated enhanced association of the Cdk5 activators and CaMKIIα. *Statistically different (p < 0.05) from control treatments; **statistically different (p < 0.05) from Glu/Gly treatment by one-way ANOVA. A, Hippocampal slices were treated with either buffer or 100 μ
m
glutamate/10 μ
m
glycine (Glu/Gly) in the absence or presence of 5 m
m
EDTA for 5 min. Crude synaptosome fractions prepared from these neurons were used for the immunoprecipitation of p35. The amounts of CaMKIIα (top) and Cdk5 (bottom) associated with p35 were analyzed by immunoblotting. Quantitative analysis from three independent experiments is presented in the histogram. The amount of CaMKIIα immunoprecipitated with p35 is presented as a percentage of the values that were measured in control treatments and is the mean ± SEM of three independent experiments. B, Hippocampal slices were treated with 100 μ
m
glutamate/10 μ
m
glycine (Glu/Gly) in the absence or presence of 5 m
m
EGTA for 5 min. The lysates were used for the immunoprecipitation of p39, and the amounts of CaMKIIα (top) and Cdk5 (bottom) associated with p39 were analyzed by immunoblotting. Quantitative analysis from three independent experiments is presented in the histogram.C, Hippocampal slices were treated with either buffer or 100 μ
m
glutamate/10 μ
m
glycine (Glu/Gly) in the presence or absence of 10 μ
m
each KN62, KN93, and KN92 or 0.5 μ
m
H-89 for 5 min. Crude synaptosome fractions prepared from these neurons were used for the immunoprecipitation of p35. The amounts of CaMKIIα (top) and Cdk5 (bottom) associated with p35 were analyzed by immunoblotting. Quantitative analysis from three independent experiments is presented in the _middle_histogram. The amount of CaMKIIα immunoprecipitated with p35 is presented as a percentage of the values that were measured in control treatments and is the mean ± SEM of three independent experiments. In the bottom histogram crude synaptosome fractions prepared from the treated slices described above were assayed for total and Ca2+/CaM-independent CaMKII kinase activity (see Materials and Methods). Autonomous CaMKII activity is defined as the Ca2+/CaM-independent activity expressed as a percentage of the total CaMKII activity. The autonomous CaMKII activity in slices is expressed as a percentage of the autonomous CaMKII activity in control slices and is the mean ± SEM from three independent experiments.
Fig. 7.
Activation of the NMDA receptor enhances the association of CaMKIIα and p35. Hippocampal slices were treated with either buffer or 100 μ
m
glutamate/10 μ
m
glycine in the absence (Glu) or presence of 10 μ
m
MK801 (Glu/MK801), 100 μ
m
CNQX (Glu/CNQX), 100 μ
m
AIDA (Glu/AIDA), and 10 μ
m
MK801 plus 100 μ
m
CNQX plus 100 μ
m
AIDA (Glu/MK801+CNQX+AIDA) for 5 min. Hippocampal slices also were treated with either 100 μ
m
NMDA in the absence (NMDA) or presence of 10 μ
m
MK801 (NMDA/MK801) or 10 μ
m
KN62 (NMDA/KN62) or 100 μ
m
AMPA in the absence (AMPA) or presence of 100 μ
m
CNQX (AMPA/CNQX) or 10 μ
m
KN62 (AMPA/KN62) for 5 min. Crude synaptosome fractions prepared from these neurons were used for the immunoprecipitation of p35. The amounts of CaMKIIα (top) and Cdk5 (bottom) associated with p35 were analyzed by immunoblotting. Quantitative analysis from three independent experiments is presented in the histogram. The amount of CaMKIIα immunoprecipitated with p35 is presented as a percentage of the values that were measured in control treatments and is the mean ± SEM of three independent experiments. aStatistically different (p < 0.05) from control treatment;bstatistically different (p < 0.05) from Glu treatment; cstatistically different (p < 0.05) from NMDA treatment;dstatistically different (p < 0.05) from AMPA treatment by one-way ANOVA.
Similar articles
- Multivalent interactions of calcium/calmodulin-dependent protein kinase II with the postsynaptic density proteins NR2B, densin-180, and alpha-actinin-2.
Robison AJ, Bass MA, Jiao Y, MacMillan LB, Carmody LC, Bartlett RK, Colbran RJ. Robison AJ, et al. J Biol Chem. 2005 Oct 21;280(42):35329-36. doi: 10.1074/jbc.M502191200. Epub 2005 Aug 24. J Biol Chem. 2005. PMID: 16120608 - Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin.
Walikonis RS, Oguni A, Khorosheva EM, Jeng CJ, Asuncion FJ, Kennedy MB. Walikonis RS, et al. J Neurosci. 2001 Jan 15;21(2):423-33. doi: 10.1523/JNEUROSCI.21-02-00423.2001. J Neurosci. 2001. PMID: 11160423 Free PMC article. - Enhanced activation of Ca2+/calmodulin-dependent protein kinase II upon downregulation of cyclin-dependent kinase 5-p35.
Hosokawa T, Saito T, Asada A, Ohshima T, Itakura M, Takahashi M, Fukunaga K, Hisanaga S. Hosokawa T, et al. J Neurosci Res. 2006 Sep;84(4):747-54. doi: 10.1002/jnr.20975. J Neurosci Res. 2006. PMID: 16802322 - The regulation of cyclin-dependent kinase 5 activity through the metabolism of p35 or p39 Cdk5 activator.
Hisanaga S, Saito T. Hisanaga S, et al. Neurosignals. 2003 Sep-Oct;12(4-5):221-9. doi: 10.1159/000074624. Neurosignals. 2003. PMID: 14673209 Review. - The protein kinase Cdk5. Structural aspects, roles in neurogenesis and involvement in Alzheimer's pathology.
Maccioni RB, Otth C, Concha II, Muñoz JP. Maccioni RB, et al. Eur J Biochem. 2001 Mar;268(6):1518-27. doi: 10.1046/j.1432-1033.2001.02024.x. Eur J Biochem. 2001. PMID: 11248668 Review.
Cited by
- Identification of a novel, membrane-associated neuronal kinase, cyclin-dependent kinase 5/p35-regulated kinase.
Kesavapany S, Lau KF, Ackerley S, Banner SJ, Shemilt SJ, Cooper JD, Leigh PN, Shaw CE, McLoughlin DM, Miller CC. Kesavapany S, et al. J Neurosci. 2003 Jun 15;23(12):4975-83. doi: 10.1523/JNEUROSCI.23-12-04975.2003. J Neurosci. 2003. PMID: 12832520 Free PMC article. - Cyclin-dependent kinase 5 activity regulates pain signaling.
Pareek TK, Keller J, Kesavapany S, Pant HC, Iadarola MJ, Brady RO, Kulkarni AB. Pareek TK, et al. Proc Natl Acad Sci U S A. 2006 Jan 17;103(3):791-6. doi: 10.1073/pnas.0510405103. Epub 2006 Jan 9. Proc Natl Acad Sci U S A. 2006. PMID: 16407116 Free PMC article. - Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis.
Amin ND, Zheng YL, Kesavapany S, Kanungo J, Guszczynski T, Sihag RK, Rudrabhatla P, Albers W, Grant P, Pant HC. Amin ND, et al. J Neurosci. 2008 Apr 2;28(14):3631-43. doi: 10.1523/JNEUROSCI.0453-08.2008. J Neurosci. 2008. PMID: 18385322 Free PMC article. - NMDA receptor regulates migration of newly generated neurons in the adult hippocampus via Disrupted-In-Schizophrenia 1 (DISC1).
Namba T, Ming GL, Song H, Waga C, Enomoto A, Kaibuchi K, Kohsaka S, Uchino S. Namba T, et al. J Neurochem. 2011 Jul;118(1):34-44. doi: 10.1111/j.1471-4159.2011.07282.x. Epub 2011 May 19. J Neurochem. 2011. PMID: 21517847 Free PMC article. - Inducible Knockout of the Cyclin-Dependent Kinase 5 Activator p35 Alters Hippocampal Spatial Coding and Neuronal Excitability.
Kamiki E, Boehringer R, Polygalov D, Ohshima T, McHugh TJ. Kamiki E, et al. Front Cell Neurosci. 2018 May 17;12:138. doi: 10.3389/fncel.2018.00138. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29867369 Free PMC article.
References
- Bayer KU, Schulman H. Regulation of signal transduction by protein targeting: the case for CaMKII. Biochem Biophys Res Commun. 2001;289:917–923. - PubMed
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. - PubMed
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by cdk5 modulates dopamine signaling in neurons. Nature. 1999;402:669–671. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous