Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius - PubMed (original) (raw)
Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius
Mark W Doyle et al. J Neurosci. 2002.
Abstract
Although the central terminals of cranial visceral afferents express vanilloid receptor 1 (VR1), little is known about their functional properties at this first synapse within the nucleus tractus solitarius (NTS). Here, we examined whether VR1 modulates afferent synaptic transmission. In horizontal brainstem slices, solitary tract (ST) activation evoked EPSCs. Monosynaptic EPSCs had low synaptic jitter (SD of latency to successive shocks) averaging 84.03 +/- 3.74 microsec (n = 72) and were completely blocked by the non-NMDA antagonist 2,3-dihydroxy-6-nitro-7-sulfonyl-benzo[f]quinoxaline (NBQX). Sustained exposure to the VR1 agonist capsaicin (CAP; 100 nm) blocked ST EPSCs (CAP-sensitive) in some neurons but not others (CAP-resistant). CAP-sensitive EPSCs had longer latencies than CAP-resistant EPSCs (4.65 +/- 0.27 msec, n = 48 vs 3.53 +/- 0.28 msec, n = 24, respectively; p = 0.011), but they had similar jitter. CAP evoked two transient responses in CAP-sensitive neurons: a rapidly developing inward current (I(cap)) (108.1 +/- 22.9 pA; n = 21) and an increase in spontaneous synaptic activity. After 3-5 min in CAP, I(cap) subsided and ST EPSCs disappeared. NBQX completely blocked I(cap). The VR1 antagonist capsazepine (10-20 microm) attenuated CAP responses. Anatomically, second-order NTS neurons were identified by 4-(4-dihexadecylamino)styryl)-N-methylpyridinium iodide transported from the cervical aortic depressor nerve (ADN) to stain central terminals. Neurons with fluorescent ADN contacts had CAP-sensitive EPSCs (n = 5) with latencies and jitter similar to those of unlabeled monosynaptic neurons. Thus, consistent with presynaptic VR1 localization, CAP selectively activates a subset of ST axons to release glutamate that acts on non-NMDA receptors. Because the CAP sensitivity of cranial afferents is exclusively associated with unmyelinated axons, VR1 identifies C-fiber afferent pathways within the brainstem.
Figures
Fig. 1.
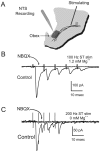
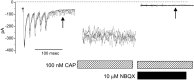
ST-evoked NTS synaptic responses in horizontal brainstem slices. A, Orientation of the rat brainstem slices in the horizontal plane allowed placement of the concentric bipolar stimulating electrode on the ST several millimeters from the recording region (dark gray) in the mNTS.B, ST activation evoked monosynaptic EPSCs in an mNTS neuron. _V_M = −70 mV. In the control, each ST shock (train of 5 pulses at 100 Hz) evoked a short-latency EPSC with high reliability (latency, 1.9 msec; jitter, 38 μsec; no observed failures). The latency was averaged over 50 sweeps. Synaptic jitter was calculated as the SD of these trial latencies. Successive shocks evoked a frequency-dependent depression of EPSC amplitude. NBQX (50 μ
m
) completely blocked the EPSCs (flat trace). Note that this neuron possessed fluorescently labeled baroreceptor contacts and thus was anatomically monosynaptic to the ST (Fig. 7_A_, inset). C, In another neuron, ST evoked EPSCs with no NMDA current component in nominally Mg2+-free solution. NBQX completely blocked this highly reliable EPSC (latency, 2.3 msec; jitter, 46 μsec; no observed failures at 200 Hz ST stimulation).
Fig. 2.
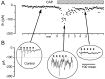
Responses of a representative CAP-sensitive mNTS neuron. A, _I_h measured each 4 sec at −70 mV were small and constant during the control period. Application of 100 n
m
CAP (bar) evoked an_I_cap, which developed over 10–20 sec and then subsided within 5 min in the continued presence of CAP.B, Bursts of ST stimulation evoked bursts of EPSCs in these neurons under control conditions; these evoked EPSCs were blocked by CAP. Expanded time segments from A show the train of EPSCs evoked by five ST shocks (solid circles), and these were diminished at the peak of _I_cap(middle trace) when spontaneous synaptic activity increased. After 5 min in 100 n
m
CAP,I_cap subsided and ST stimulation no longer evoked synaptic responses (bottom right trace). Synaptic responses in this cell met monosynaptic reliability criteria (latency, 3.12 msec; jitter, 120 μsec; no observable failures). The_dotted line is a reference set at the approximate level of the peak _I_cap.
Fig. 3.
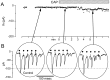
CAP had no effect on ST synaptic transmission in a CAP-resistant mNTS neuron. A, ST evoked EPSCs in a CAP-resistant neuron. Synaptic responses in this cell met monosynaptic reliability criteria (latency, 4.7 msec; jitter, 107 μsec; no observable failures). Such responses showed typical amplitude depression to high-frequency trains of ST shocks. The gaps in data points during control are missing points. B, Bath application of CAP (100 n
m
) failed to alter the amplitude of the evoked EPSCs or to change the _I_h(data not shown). The dotted line is a reference set at 100 pA.
Fig. 4.
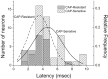
Histograms plot the distribution of latency values for CAP-sensitive (solid line) and CAP-resistant (dashed line) EPSCs of mNTS neurons. The mean latency for CAP-sensitive neurons was significantly longer than for CAP-resistant neurons (unpaired t test;p = 0.011; 4.65 ± 0.27 msec,n = 48 vs 3.53 ± 0.28 msec,n = 24, respectively). Solid lines(CAP-sensitive) and dashed lines (CAP-resistant) represent normal distributions.
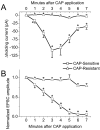
Fig. 5.
Average time course of the changes in_I_h and amplitude of ST-evoked EPSCs in CAP-sensitive and CAP-resistant second-order neurons. A, CAP application significantly increased the_I_h in CAP-sensitive compared with CAP-resistant neurons (n = 21 and 19, respectively;p < 0.0001; RM ANOVA; error bars indicate SEM). Individual time point comparisons were made with one-way ANOVA (*p ≤ 0.0174). B, CAP inhibited the EPSC amplitude significantly in CAP-sensitive compared with CAP-resistant neurons (n = 21 and 19, respectively;p < 0.0001; RM ANOVA; error bars indicate SEM). Individual time point comparisons were made with one-way ANOVA (*p ≤ 0.0222).
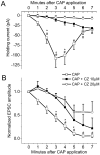
Fig. 6.
The VR1 antagonist capsazepine (CZ) inhibited the effects of CAP. All neurons were CAP-sensitive.A, Both 10 and 20 μ
m
CZ significantly (each n = 4) reduced _I_hchanges in response to 100 n
m
CAP (n = 21; p = 0.0131; RM ANOVA). Individual time point comparisons of CAP alone, CAP plus 10 μ
m
capsazepine, and CAP plus 20 μ
m
capsazepine by one-way ANOVA with Fisher's PLSD post hoc analysis are shown (*p < 0.05 between CAP alone and CAP plus 10 μ
m
capsazepine; #p < 0.05 between CAP alone and CAP plus 20 μ
m
capsazepine; error bars indicate SEM). B, Both 10 and 20 μ
m
capsazepine inhibited and delayed the reduction of the evoked EPSC amplitude (p = 0.0007; RM ANOVA). Individual time point comparisons of EPSC in CAP alone, CAP plus 10 μ
m
capsazepine, and CAP plus 20 μ
m
capsazepine by one-way ANOVA with Fisher's PLSD post hoc analysis are shown (*p < 0.05 between CAP alone and CAP plus 10 μ
m
capsazepine;#p < 0.05 between CAP alone and CAP plus 20 μ
m
capsazepine; error bars indicate SEM).
Fig. 7.
CAP response recorded in an mNTS neuron with an anatomically identified sensory input. The DiA fluorescent image of ADN terminals (inset, blue) was superimposed on the infrared DIC image of this mNTS neuron. Fluorescent DiA labeling of aortic baroreceptor afferents was found primarily on cell bodies and indicated directly that these neurons received visceral sensory contacts. The outline of the neuron in the DIC focal plane is apparent with the associated fluorescently labeled contacts. ST stimulation-evoked EPSCs met monosynaptic latency criteria. CAP (100 n
m
, red bar) evoked a transient _I_cap similar to unlabeled NTS neurons. _I_cap peaked in 1–2 min and subsided to control levels within 5 min in the continued presence of CAP.
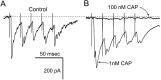
Fig. 8.
Low concentrations of CAP enhanced ST-evoked EPSCs. A, In control solutions, ST stimulation evoked a train of EPSCs. B, After 3 min in 1 n
m
CAP, identical trains of ST stimuli evoked larger EPSCs, with no change in the _I_h. Higher concentrations of CAP (100 n
m
) blocked the EPSCs (flat trace). The dotted line indicates the control level of the_I_h. All traces are the means of five consecutive responses to ST stimulus trains containing bursts of five shocks.
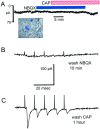
Fig. 9.
A, The non-NMDA antagonist NBQX (50 μ
m
) prevented I_cap in a baroreceptor-labeled mNTS neuron (inset). ST-evoked EPSCs in this neuron met the reliability criteria for monosynaptic responses (see control response traces for this neuron in Fig. 1_B). NBQX blocked all ST-evoked synaptic activity and prevented _I_cap in response to 100 n
m
CAP. B, CAP continued to block ST-evoked EPSCs after the removal of NBQX, demonstrating CAP sensitivity in this neuron. C, Evoked EPSCs returned after the removal of CAP, indicating intact postsynaptic receptors.
Fig. 10.
Non-NMDA receptors were functional during CAP. Spontaneous EPSCs occurred unsynchronized to ST electrical stimulation before CAP application (left, arrow), and this activity was relatively high in this neuron. During 100 n
m
CAP application, the _I_h(_V_H = −70 mV) increased, as did the frequency of spontaneous EPSCs (middle, hatched bar). ST-triggered EPSCs were blocked by CAP. Application of NBQX (10 μ
m
) during _I_capeliminated changes in _I_h and blocked spontaneous synaptic activity (right,arrow). These results are consistent with dependence on non-NMDA receptors for ST EPSCs, spontaneous EPSCs, and_I_cap. Because spontaneous EPSCs persisted in CAP, they appear to arise from CAP-resistant sources (A-type or nonafferent contacts). Each panel displays five consecutive, superimposed sweeps during 50 Hz of ST stimulation. Each train was separated by 2 sec. Dotted line indicates the zero_I_h level for reference.
Fig. 11.
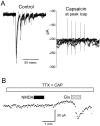
Glutamate (Glu) receptors were functional during CAP treatment. A, A 100 n
m
concentration of CAP increased the spontaneous synaptic activity (note the spontaneous synaptic currents in the initial segment of overlaid traces) and blocked ST-evoked monosynaptic EPSCs (latency, 6.12 msec; jitter, 82 μsec). B, After_I_cap subsided and in the presence of CAP and TTX, a brief application of NMDA (2 m
m
) had no effect on the _I_h, but a brief application of glutamate (1 m
m
) rapidly induced a large_I_cap consistent with intact non-NMDA receptors.
Similar articles
- Vanilloid-sensitive afferents activate neurons with prominent A-type potassium currents in nucleus tractus solitarius.
Bailey TW, Jin YH, Doyle MW, Andresen MC. Bailey TW, et al. J Neurosci. 2002 Sep 15;22(18):8230-7. doi: 10.1523/JNEUROSCI.22-18-08230.2002. J Neurosci. 2002. PMID: 12223577 Free PMC article. - Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius.
Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Jin YH, et al. J Neurosci. 2004 May 19;24(20):4709-17. doi: 10.1523/JNEUROSCI.0753-04.2004. J Neurosci. 2004. PMID: 15152030 Free PMC article. - Comparison of baroreceptive to other afferent synaptic transmission to the medial solitary tract nucleus.
Andresen MC, Peters JH. Andresen MC, et al. Am J Physiol Heart Circ Physiol. 2008 Nov;295(5):H2032-42. doi: 10.1152/ajpheart.00568.2008. Epub 2008 Sep 12. Am J Physiol Heart Circ Physiol. 2008. PMID: 18790834 Free PMC article. - Differentiation of autonomic reflex control begins with cellular mechanisms at the first synapse within the nucleus tractus solitarius.
Andresen MC, Doyle MW, Bailey TW, Jin YH. Andresen MC, et al. Braz J Med Biol Res. 2004 Apr;37(4):549-58. doi: 10.1590/s0100-879x2004000400012. Epub 2004 Mar 23. Braz J Med Biol Res. 2004. PMID: 15064818 Review. - The unsilent majority-TRPV1 drives "spontaneous" transmission of unmyelinated primary afferents within cardiorespiratory NTS.
Andresen MC, Hofmann ME, Fawley JA. Andresen MC, et al. Am J Physiol Regul Integr Comp Physiol. 2012 Dec 15;303(12):R1207-16. doi: 10.1152/ajpregu.00398.2012. Epub 2012 Oct 17. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 23076872 Free PMC article. Review.
Cited by
- Neural circuits of long-term thermoregulatory adaptations to cold temperatures and metabolic demands.
Mota CMD, Madden CJ. Mota CMD, et al. Nat Rev Neurosci. 2024 Mar;25(3):143-158. doi: 10.1038/s41583-023-00785-8. Epub 2024 Feb 5. Nat Rev Neurosci. 2024. PMID: 38316956 Review. - Transient receptor potential channels as an emerging therapeutic target for oropharyngeal dysphagia.
Hossain MZ, Kitagawa J. Hossain MZ, et al. Jpn Dent Sci Rev. 2023 Dec;59:421-430. doi: 10.1016/j.jdsr.2023.09.002. Epub 2023 Nov 10. Jpn Dent Sci Rev. 2023. PMID: 38022386 Free PMC article. Review. - CCK-sensitive C fibers activate NTS leptin receptor-expressing neurons via NMDA receptors.
Neyens DM, Brenner L, Calkins R, Winzenried ET, Ritter RC, Appleyard SM. Neyens DM, et al. Am J Physiol Regul Integr Comp Physiol. 2024 May 1;326(5):R383-R400. doi: 10.1152/ajpregu.00238.2022. Epub 2023 Dec 18. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 38105761 Free PMC article. - Interleukin-1β and interleukin-6 enhance thermal prolongation of the LCR in decerebrate piglets.
Xia L, Bartlett D Jr, Leiter JC. Xia L, et al. Respir Physiol Neurobiol. 2016 Aug;230:44-53. doi: 10.1016/j.resp.2016.05.006. Epub 2016 May 12. Respir Physiol Neurobiol. 2016. PMID: 27181326 Free PMC article. - Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways.
Starowicz K, Maione S, Cristino L, Palazzo E, Marabese I, Rossi F, de Novellis V, Di Marzo V. Starowicz K, et al. J Neurosci. 2007 Dec 12;27(50):13739-49. doi: 10.1523/JNEUROSCI.3258-07.2007. J Neurosci. 2007. PMID: 18077685 Free PMC article.
References
- Andresen MC, Kunze DL. Nucleus tractus solitarius: gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. - PubMed
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–H1311. - PubMed
- Andresen MC, Krauhs JM, Brown AM. Relationship of aortic wall baroreceptor properties during development in normotensive and spontaneously hypertensive rats. Circ Res. 1978;43:728–738. - PubMed
- Andresen MC, Doyle MW, Jin Y-H, Bailey TW. Cellular mechanisms of baroreceptor integration at the nucleus tractus solitarius. Ann NY Acad Sci. 2001;940:132–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous