The alternative sigma factor sigma(E) plays an important role in intestinal survival and virulence in Vibrio cholerae - PubMed (original) (raw)
The alternative sigma factor sigma(E) plays an important role in intestinal survival and virulence in Vibrio cholerae
Gabriela Kovacikova et al. Infect Immun. 2002 Oct.
Abstract
The alternative sigma factor sigma(E) (RpoE) is involved in the response to extracytoplasmic stress and plays a role in the virulence of a variety of different bacteria. To assess the role of sigma(E) in Vibrio cholerae pathogenesis, a DeltarpoE mutant was constructed and analyzed using the infant mouse model. The results here show that sigma(E) contributes significantly to the virulence of V. cholerae. The DeltarpoE mutant was highly attenuated with a 50% lethal dose more than 3 logs higher than that for the parental strain, and its ability to colonize the intestine was reduced approximately 30-fold. A time course of infection revealed that the number of CFU of the DeltarpoE mutant was approximately 1 log lower than that of the parental strain by 12 h postinoculation and decreased further by 24 h. The defect in virulence in the DeltarpoE mutant thus appears to be a diminished ability to survive within the intestinal environment. The results here also show that sigma(E) is not required for growth and survival of V. cholerae in vitro at high temperatures but is required under other stressful conditions, such as in the presence of 3% ethanol. As in Escherichia coli, the expression of rpoE in V. cholerae is dependent upon two promoters located upstream of the gene, P1 and P2. P1 appears to be sigma(70) dependent, whereas the downstream promoter, P2, is positively autoregulated by sigma(E).
Figures
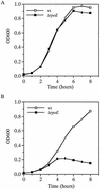
FIG. 1.
Growth of O395 and the Δ_rpoE_ derivative KSK1665 in LB medium at 43°C (A) and in the presence of 3% ethanol at 37°C (B). Open boxes, O395 (wild type wt]); closed boxes, KSK1665.
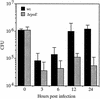
FIG. 2.
CFU of CG842 and the Δ_rpoE_ derivative KSK1665 at various times postinoculation from infant mice. Black bars, CG842; grey bars, KSK1665. Using the Prism program, a nonpaired two-tailed t test with the values from the 24-h time point were statistically significant (P = 0.01). Error bars, standard deviations.
FIG. 3.
Organization of the V. cholerae rpoE P1 and P2 promoters. (A) The distances between the transcriptional start sites and the rpoE start codon (not to scale) are shown. (B) The nucleotide sequences of the −35 and −10 regions of the promoters are shown. The base pairs within the E. coli rpoE P2 −35 and −10 regions that are identical to those of the V. cholerae rpoE P2 promoter are shown in bold.
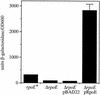
FIG. 4.
Autoregulation of rpoE expression in V. cholerae. Cultures were grown in LB medium, pH 6.5, at 30°C. From left to right, strains are KSK1686 (rpoE+), KSK1695 (Δ_rpoE_), KSK1695 pBAD22, and KSK1695 pGKK220 (pRpoE). The cultures with plasmids contained 0.05% arabinose. Error bars, standard deviations.
FIG. 5.
Construction of the chromosomal rpoE ΔP1 and ΔP2 mutations. PCR was carried out with oppositely oriented overlapping primers which flank the −35 and −10 regions of the rpoE P1 and P2 promoters and which contain restriction sites for the type IIS enzyme _Ear_I. For the P1 promoter deletion mutant (top), SE-P11 and SE-Eco produced a 150-bp upstream fragment (black bar) and SE-P12 and SE-NotI produced a 320-bp downstream fragment (grey bar). For the P2 promoter deletion mutant (bottom), SE-P21 and SE-Eco produced a 370-bp upstream fragment (black bar) and SE-P22 and SE-NotI produced a 100-bp downstream fragment (grey bar). For each promoter, ligation of the two resulting fragments together into pKAS32 generated deletions of 31 bp. A promoterless lacZ gene was then introduced into these constructs in the proper orientation, and the fusions were transferred into pKAS180 prior to allelic exchange into CG842.
References
- Chiang, S. L., R. K. Taylor, M. Koomey, and J. J. Mekalanos. 1995. Single amino acid substitutions in the N terminus of Vibrio cholerae TcpA affect colonization, autoagglutination, and serum resistance. Mol. Microbiol. 17:1133-1142. - PubMed
- Cotter, P. A., and V. J. DiRita. 2000. Bacterial virulence gene regulation: an evolutionary perspective. Annu. Rev. Microbiol. 54:519-565. - PubMed
- De Las Peñas, A., L. Connolly, and C. A. Gross. 1997. The σΕ−mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of σΕ. Mol. Microbiol. 24:373-385. - PubMed
- de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386-405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials