Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes - PubMed (original) (raw)
Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes
Pierre Vaudaux et al. Infect Immun. 2002 Oct.
Abstract
Small colony variants (SCVs) of Staphylococcus aureus are slow-growing subpopulations that cause persistent and relapsing infections. The altered phenotype of SCV can arise from defects in menadione or hemin biosynthesis, which disrupt the electron transport chain and decrease ATP concentrations. With SCVs, virulence is altered by a decrease in exotoxin production and susceptibility to various antibiotics, allowing their intracellular survival. The expression of bacterial adhesins by SCVs is poorly documented. We tested fibrinogen- and fibronectin-mediated adhesion of a hemB mutant of S. aureus 8325-4 that is defective for hemin biosynthesis and exhibits a complete SCV phenotype. In this strain, adhesion to fibrinogen and fibronectin was significantly higher than that of its isogenic, normally growing parent and correlated with the increased surface display of these adhesins as assessed by flow cytometry. Real-time quantitative reverse transcription-PCR demonstrated increased expression of clfA and fnb genes by the hemB mutant compared to its isogenic parent. The influence of the hemB mutation on altered adhesin expression was confirmed by showing complete restoration of the wild-type adhesive phenotype in the hemB mutant, either by complementing with intact hemB or by supplementing the growth medium with hemin. Increased surface display of fibrinogen and fibronectin adhesins by the hemB mutation occurred independently from agr, a major regulatory locus of virulence factors in S. aureus. Both agr-positive and agr-lacking hemB mutants were also more efficiently internalized by human embryonic kidney cells than were their isogenic controls, presumably because of increased surface display of their fibronectin adhesins.
Figures
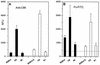
FIG. 1.
Adhesion to fibrinogen-coated (A) or fibronectin-coated (B) coverslips of the parental strain (8325-4), its hemB mutant (strain I10) with (1 μg/ml) or without supplementation with hemin, and the pCE12-complemented mutant (strain A2) with 0.5% xylose in MHB.
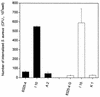
FIG. 2.
Adhesion to fibrinogen-coated (A) or fibronectin-coated (B) coverslips of the parental RNAIII-defective strain (8325-4G), its hemB mutant (strain I15), and the pCE12-complemented mutant (strain K1) with 0.5% xylose in MHB.
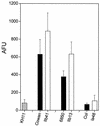
FIG. 3.
Binding of soluble anti-ClfA antibodies (A) or FITC-labeled fibronectin (Fn-FITC) (B) by strain 8325-4 or its hemB (strain I10) or pCE12-complemented (strain A2) hemB mutants was compared to that of the RNAIII-defective strain 8325-4G and its respective hemB (strain I15) or pCE12-complemented (strain K1) hemB mutants. Dashed lines represent the baseline binding of anti-ClfA and Fn-FITC by clfA mutant DU5880 (A) and fnbA fnbB mutant DU5883 (B) strains, respectively. Results are means + SEM (error bars) of three experiments performed in duplicate and scored in arbitrary fluorescence units (AFU).
FIG. 4.
Increased uptake of SCV hemB mutants by human embryonic kidney cells, scored as the number of internalized (lysostaphin-protected) bacteria. Internalization of strain 8325-4 or its hemB (strain I10) or pCE12-complemented (strain A2) hemB mutants was compared to that of the RNAIII-defective strain 8325-4G and its respective hemB (strain I15) or pCE12-complemented (strain K1) hemB mutants. Results are presented as means + SEM (error bars)
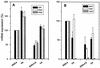
FIG. 5.
mRNA expression of clfA, fnbA, and fnbB (A) or sarA, rnaII, and rnaIII (B) genes determined by real-time RT-PCR of 16S rRNA adjusted extracts. Data are presented as means + SEM (error bars) of three experiments performed in triplicate.
FIG. 6.
Binding of soluble FITC-labeled fibronectin (Fn-FITC) by strain Cowan and its hemB mutant IIb41, 6850 and its hemB mutant IIb13, and MRSA COL and its hemB mutant Ia48, compared to S. epidermidis strain KH11 taken as a negative control of FnBP display. Results are presented as means + SEM (error bars) of four experiments performed in duplicate and scored in arbitrary fluorescence units (AFU).
Similar articles
- A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly.
von Eiff C, Heilmann C, Proctor RA, Woltz C, Peters G, Götz F. von Eiff C, et al. J Bacteriol. 1997 Aug;179(15):4706-12. doi: 10.1128/jb.179.15.4706-4712.1997. J Bacteriol. 1997. PMID: 9244256 Free PMC article. - Heme-Dependent Siderophore Utilization Promotes Iron-Restricted Growth of the Staphylococcus aureus hemB Small-Colony Variant.
Batko IZ, Flannagan RS, Guariglia-Oropeza V, Sheldon JR, Heinrichs DE. Batko IZ, et al. J Bacteriol. 2021 Nov 19;203(24):e0045821. doi: 10.1128/JB.00458-21. Epub 2021 Oct 4. J Bacteriol. 2021. PMID: 34606375 Free PMC article. - Rot and Agr system modulate fibrinogen-binding ability mainly by regulating clfB expression in Staphylococcus aureus NCTC8325.
Xue T, You Y, Shang F, Sun B. Xue T, et al. Med Microbiol Immunol. 2012 Feb;201(1):81-92. doi: 10.1007/s00430-011-0208-z. Epub 2011 Jun 24. Med Microbiol Immunol. 2012. PMID: 21701848 - Staphylococcus aureus Small Colony Variants (SCVs): a road map for the metabolic pathways involved in persistent infections.
Proctor RA, Kriegeskorte A, Kahl BC, Becker K, Löffler B, Peters G. Proctor RA, et al. Front Cell Infect Microbiol. 2014 Jul 28;4:99. doi: 10.3389/fcimb.2014.00099. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25120957 Free PMC article. Review. - Small colony variants of Staphylococcus aureus--review.
Melter O, Radojevič B. Melter O, et al. Folia Microbiol (Praha). 2010 Nov;55(6):548-58. doi: 10.1007/s12223-010-0089-3. Epub 2011 Jan 21. Folia Microbiol (Praha). 2010. PMID: 21253898 Review.
Cited by
- The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus.
Foster TJ. Foster TJ. Eur J Clin Microbiol Infect Dis. 2016 Dec;35(12):1923-1931. doi: 10.1007/s10096-016-2763-0. Epub 2016 Sep 7. Eur J Clin Microbiol Infect Dis. 2016. PMID: 27604831 Review. - Physiological characterization of a heme-deficient mutant of Staphylococcus aureus by a proteomic approach.
Kohler C, von Eiff C, Peters G, Proctor RA, Hecker M, Engelmann S. Kohler C, et al. J Bacteriol. 2003 Dec;185(23):6928-37. doi: 10.1128/JB.185.23.6928-6937.2003. J Bacteriol. 2003. PMID: 14617657 Free PMC article. - Escherichia coli variants in periprosthetic joint infection: diagnostic challenges with sessile bacteria and sonication.
Sendi P, Frei R, Maurer TB, Trampuz A, Zimmerli W, Graber P. Sendi P, et al. J Clin Microbiol. 2010 May;48(5):1720-5. doi: 10.1128/JCM.01562-09. Epub 2010 Mar 24. J Clin Microbiol. 2010. PMID: 20335421 Free PMC article. - In vitro evaluation of CBR-2092, a novel rifamycin-quinolone hybrid antibiotic: microbiology profiling studies with staphylococci and streptococci.
Robertson GT, Bonventre EJ, Doyle TB, Du Q, Duncan L, Morris TW, Roche ED, Yan D, Lynch AS. Robertson GT, et al. Antimicrob Agents Chemother. 2008 Jul;52(7):2324-34. doi: 10.1128/AAC.01651-07. Epub 2008 Apr 28. Antimicrob Agents Chemother. 2008. PMID: 18443106 Free PMC article.
References
- Arvidson, S. 2000. Extracellular enzymes, p. 379-391. In V. A. Fischetti, R. P. Novick, J. J. Ferreti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, D.C.
- Baddour, L. M., W. A. Simpson, J. J. Weems, M. M. Hill, Jr., and G. D. Christensen. 1988. Phenotypic selection of small-colony variant forms of Staphylococcus epidermidis in the rat model of endocarditis. J. Infect. Dis. 157:757-763. - PubMed
- Balwit, J. M., P. Van Langevelde, J. M. Vann, and R. A. Proctor. 1994. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J. Infect. Dis. 170:1033-1037. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources