A push-pull approach to maximize vaccine efficacy: abrogating suppression with an IL-13 inhibitor while augmenting help with granulocyte/macrophage colony-stimulating factor and CD40L - PubMed (original) (raw)
A push-pull approach to maximize vaccine efficacy: abrogating suppression with an IL-13 inhibitor while augmenting help with granulocyte/macrophage colony-stimulating factor and CD40L
Jeffrey D Ahlers et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Although a role for CD4(+) helper cells in CD8(+) cytotoxic T lymphocyte (CTL) induction by vaccines is widely recognized, much less is known about a counterbalancing role of CD4(+) T cells in down-modulating this response, or about ways to optimize vaccine responses through abrogation of this negative regulatory mechanism. Here, we discovered a synergistic enhancement of vaccine-mediated CTL induction and protection by the relief of suppression through depletion of regulatory CD4(+) cells, including CD4(+) NKT cells, or blockade of IL-13 made by these cells, combined with the cytokine granulocyte/macrophage colony-stimulating factor and the costimulatory molecule CD40L. Indeed, in the absence of helper epitopes, granulocyte/macrophage colony-stimulating factor and the helper-mimetic molecule CD40L are not sufficient to replace help to induce CTL without abrogation of CD4(+) T cell-mediated suppression, suggesting a role for T cell help in overcoming suppression. The increased CTL induction translated to striking protection against viral infection by a vaccine by using this synergistic combined approach. These results argue for a push-pull approach to maximize vaccine efficacy, especially for HIV and cancer.
Figures
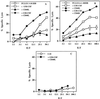
Figure 1
GM-CSF and CD40L trimer synergize for induction of CD8+CTL. (A) Specific lysis of P18IIIB (0.5 μM) coated target cells by splenic effectors from BALB/c mice immunized s.c. with 20 nmol (17 μg CTL epitope P18IIIB) HIV-1 Th-CTL vaccine construct, PCLUS 3–18IIIB, with or without individual or a combination of factors GM-CSF (5 μg/mouse), and CD40L (15 μg/mouse), incorporated in adjuvant montanide-ISA 51. Spleen cells are pooled from two mice. Error bars < the symbols are not shown. (B) CTL response is significantly (P < 0.05) enhanced by GM-CSF and CD40L after boosting with HIV Th-CTL vaccine construct, PCLUS 6.1–18IIIB. Similar results were obtained in four additional experiments. (C) Mice immunized with the minimum CTL epitope, I-10, plus GM-CSF and CD40L failed to elicit a CTL response, even after boosting and with a 4-fold higher peptide dose (80 nmol/mouse) (≈80 μg).
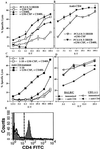
Figure 2
CD4+ cell depletion enhances CD8+ CTL response to HIV-1 Th-CTL vaccine and minimum CTL epitope. (A) Specific lysis of P18IIIB (0.5 μM) coated targets by splenic effectors after 7 days in vitro restimulation with P18IIIB and IL-2 after two s.c. immunizations with PCLUS 3–18IIIB alone, or plus individual or a combination of factors GM-CSF (5 μg/dose) and CD40L (10 μg/dose) 2 weeks apart. (B) Two additional groups of animals from the experiment in A were treated with anti-CD4 (GK1.5, 0.5 mg/day per mouse) 1 day before immunization and for 2 additional days and spleen cells harvested 15 days later, restimulated in vitro, and assayed for CTL. (C) Specific lysis by splenic effectors from mice immunized with minimum CTL epitope I-10 (60 nmol/mouse), alone, or I-10 plus GM-CSF and CD40L, restimulated in vitro with P18IIIB (0.5 μM) plus IL-2 and assayed for CTL 7 days later. Filled symbols represent mice treated with anti-CD4. Error bars < the symbols are not shown. This experiment was performed four times with comparable results. (D) CTL response of BALB/c and CD1-deficient mice after two immunizations with PCLUS 6.1–18IIIB plus GM-CSF (5 μg/dose) and CD40L (10 μg/dose). One group from each strain of mice also received anti-CD4 (GK1.5, 0.5 mg/day i.p.) 1 day before and 3 days after immunization. BALB/c and CD1(−/−) mice were significantly different (P < 0.05) without but not with CD4 depletion. Error bars < symbols are not shown. This experiment was performed two additional times with comparable results. (E) Animals treated or not with anti-CD4 were immunized with PCLUS 6.1–18IIIB, plus GM-CSF and CD40L and 15 days later spleen cells pooled from two mice each were examined for CD4+ cells, gated on CD3+ cells. Total CD4+ cells in untreated mice (gray) or mice treated 4 days with anti-CD4, 0.5 mg/day (solid line) are shown.
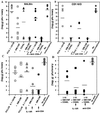
Figure 3
The combination of GM-CSF and CD40L is essential for vaccine-mediated protection from viral challenge. Female BALB/c (A) and CD1(−/−) (B) mice were immunized s.c. with 20 nmol HIV-1 vaccine PCLUS 6.1–18IIIB in ISA-51 plus indicated cytokines and costimulatory molecules and boosted i.p. Two groups immunized with PCLUS 6.1–18IIIB alone or with the combination GM-CSF and CD40L were depleted of CD4+ cells by treating with GK1.5 antibody (0.5 μg/dose i.p.) 1 day before and 3 consecutive days after immunization. Mice were challenged i.p. 5 days later with 1 × 107 pfu recombinant vaccinia expressing the HIV-1 IIIB gp160. Ovaries from individual mice were harvested 5 days after challenge, and titered on BSC-1 cells as described (25). (C) Female BALB/c mice were immunized twice with PCLUS 6.1–18IIIB with or without GM-CSF and challenged with recombinant vaccinia as in A. Mice immunized with PCLUS 6.1–18IIIB alone (open triangles), or plus GM-CSF (open diamonds), received five treatments (250 μg/mouse i.p. on day 0, 1, 2, 4, and 6 after immunization) with IL-13Rα2-Fc, an inhibitor of IL-13. Another group of animals receiving the vaccine plus GM-CSF were depleted of CD4+ cells (filled circles). (D) Female BALB/c mice were immunized twice with the minimum CTL epitope I-10 (80 nmol) with GM-CSF (5 μg/mouse) and CD40L (10 μg/mouse) and challenged with recombinant vaccinia (filled circles) as in C. Animals also received either the IL-13 inhibitor IL-13Rα2-Fc (filled squares), or anti-CD4 treatment (filled diamonds). Each symbol represents an individual animal and horizontal bars represent the geometric mean titers for each group.
Figure 4
Enhancement of vaccine elicited CD8+ CTL response and memory by relief of CD4+ cell mediated suppression by treatment with an in vivo inhibitor of IL-13. (A and D) CTL response of female BALB/c mice given a single s.c. immunization with 20 nmol vaccine construct PCLUS 6.1–18IIIB in Montanide ISA51. Animals received five doses (175 μg/dose at day 0, 1, 2, 4, 6) i.p. of either IL-13Rα2Fc (filled circles) or control human IgG (Panglobulin) (open circles). Anti-CD4 (crossed circle) (0.5 mg/day) was given 1 day before immunization and 3 consecutive days thereafter. In all six exhibits, spleen cells were restimulated in vitro with P18IIIB peptide and IL-2 for 7 days before testing in a 51Cr release assay. Error bars < the symbols are not shown. Shown is specific lysis of P18IIIB (0.5 μM)-coated target cells by spleen cell effectors from the immunized mice harvested 14 days (A) or 7 weeks (D) after a single immunization. (B and E) CTL response of female BALB/c mice given a single s.c. immunization with 20 nmol of PCLUS 6.1–18IIIB plus GM-CSF, and either IL-13Rα2Fc (filled squares), control human IgG (Panglobulin) (open squares), or anti-CD4 (crossed square) (0.5 mg/day), and spleen cells tested for lysis as in A and D harvested 14 days (B) or 7 weeks (E) after immunization. (C and F) CTL response of female BALB/c mice given a single s.c. immunization with 20 nmol of PCLUS 6.1–18IIIB and the combination of GM-CSF (5 μg/mouse) and CD40L (15 μg/mouse), incorporated in adjuvant montanide-ISA 51. Mice were treated with control Ig (open triangle) or the IL-13 inhibitor (filled triangle) or anti CD4 antibody (crossed diamond) as in A and D and spleen cells harvested 14 days (C) or 7 weeks (F) after immunization tested as above. Except in A, CTL responses are significantly different in all vaccine groups given IL-13Rα2Fc treatment (filled symbols) compared with control IgG (open symbols) (P < 0.05–0.01). Nonspecific lysis was <15% in immunized groups and completely absent in anti-CD4-treated mice.
Similar articles
- The boosting effect of co-transduction with cytokine genes on cancer vaccine therapy using genetically modified dendritic cells expressing tumor-associated antigen.
Ojima T, Iwahashi M, Nakamura M, Matsuda K, Naka T, Nakamori M, Ueda K, Ishida K, Yamaue H. Ojima T, et al. Int J Oncol. 2006 Apr;28(4):947-53. Int J Oncol. 2006. PMID: 16525645 - Effect of immunological adjuvants: GM-CSF (granulocyte-monocyte colony stimulating factor) and IL-23 (interleukin-23) on immune responses generated against hepatitis C virus core DNA vaccine.
Hartoonian C, Ebtekar M, Soleimanjahi H, Karami A, Mahdavi M, Rastgoo N, Azadmanesh K. Hartoonian C, et al. Cytokine. 2009 Apr;46(1):43-50. doi: 10.1016/j.cyto.2008.12.007. Epub 2009 Mar 10. Cytokine. 2009. PMID: 19278866 - Vaccine therapy of established tumors in the absence of autoimmunity.
Hodge JW, Grosenbach DW, Aarts WM, Poole DJ, Schlom J. Hodge JW, et al. Clin Cancer Res. 2003 May;9(5):1837-49. Clin Cancer Res. 2003. PMID: 12738742 - A push-pull vaccine strategy using Toll-like receptor ligands, IL-15, and blockade of negative regulation to improve the quality and quantity of T cell immune responses.
Berzofsky JA. Berzofsky JA. Vaccine. 2012 Jun 19;30(29):4323-7. doi: 10.1016/j.vaccine.2011.11.034. Epub 2011 Nov 21. Vaccine. 2012. PMID: 22115635 Free PMC article. Review.
Cited by
- Current view on novel vaccine technologies to combat human infectious diseases.
Matić Z, Šantak M. Matić Z, et al. Appl Microbiol Biotechnol. 2022 Jan;106(1):25-56. doi: 10.1007/s00253-021-11713-0. Epub 2021 Dec 10. Appl Microbiol Biotechnol. 2022. PMID: 34889981 Free PMC article. Review. - Implementation of Vaccinomics and In-Silico Approaches to Construct Multimeric Based Vaccine Against Ovarian Cancer.
Sufyan M, Shahid F, Irshad F, Javaid A, Qasim M, Ashfaq UA. Sufyan M, et al. Int J Pept Res Ther. 2021;27(4):2845-2859. doi: 10.1007/s10989-021-10294-w. Epub 2021 Oct 19. Int J Pept Res Ther. 2021. PMID: 34690620 Free PMC article. - Memory T cells: strategies for optimizing tumor immunotherapy.
Liu Q, Sun Z, Chen L. Liu Q, et al. Protein Cell. 2020 Aug;11(8):549-564. doi: 10.1007/s13238-020-00707-9. Epub 2020 Mar 27. Protein Cell. 2020. PMID: 32221812 Free PMC article. Review. - In silico design of a triple-negative breast cancer vaccine by targeting cancer testis antigens.
Parvizpour S, Razmara J, Pourseif MM, Omidi Y. Parvizpour S, et al. Bioimpacts. 2019;9(1):45-56. doi: 10.15171/bi.2019.06. Epub 2018 Jul 2. Bioimpacts. 2019. PMID: 30788259 Free PMC article. - Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity.
Suschak JJ, Williams JA, Schmaljohn CS. Suschak JJ, et al. Hum Vaccin Immunother. 2017 Dec 2;13(12):2837-2848. doi: 10.1080/21645515.2017.1330236. Epub 2017 Jun 12. Hum Vaccin Immunother. 2017. PMID: 28604157 Free PMC article. Review.
References
- Rowland-Jones S, Tan R, McMichael A. Adv Immunol. 1997;65:277–346. - PubMed
- Goulder P J R, Rowland-Jones S L, McMichael A J, Walker B D. AIDS. 1999;13:S121–S136. - PubMed
- Oscherwitz J, Gotch F M, Cease K B, Berzofsky J A. AIDS. 1999;13:S163–S174. - PubMed
- Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, et al. Science. 1999;283:857–860. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials