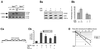Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals - PubMed (original) (raw)
Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals
Aristides G Eliopoulos et al. EMBO J. 2002.
Abstract
Macrophage activation by bacterial lipopolysaccharide (LPS) promotes the secretion of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-alpha) and interleukin-1beta (IL-1beta), and of secondary mediators, such as leukotrienes and prostaglandins (PGs). Mice lacking the gene encoding the serine/threonine protein kinase Tpl2/Cot produce low levels of TNF-alpha in response to LPS because of an ERK-dependent post-transcriptional defect, and they are resistant to LPS/D-galactosamine-induced endotoxin shock. In this study we demonstrate that prostaglandin E2 and its regulatory enzyme, COX-2, are also targets of Tpl2-transduced LPS signals in bone marrow-derived mouse macrophages. Thus, LPS-stimulated Tpl2(-/-) macrophages express low levels of COX-2 and PGE2, compared with wild-type Tpl2(+/+) cells. The ability of Tpl2 to regulate COX-2 expression depends on ERK signals that activate p90Rsk and Msk1, which in turn phosphorylate CREB, a key regulator of COX-2 transcription. These data identify physiological targets of Tpl2 signaling downstream of ERK and further implicate Tpl2 in the pathophysiology of inflammation.
Figures
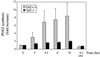
Fig. 1. LPS-treated Tpl2–/– macrophages secrete reduced levels of PGE2. Culture supernatants were collected at 0, 3, 4.5, 6 or 10 h following LPS stimulation. Unstimulated Tpl2+/+ and Tpl2–/– macrophage cultures secreted similar levels of PGE2. PGE2 levels produced by unstimulated cells were given the arbitrary value of 1. The bar graphs show the fold induction of PGE2 (± SD) compared with control untreated cultures.
Fig. 2. Tpl2 is required for optimal induction of COX-2 in LPS-stimulated macrophages. (A) Western blot of cell lysates of unstimulated and LPS-stimulated macrophages probed with antibodies against COX-2 (upper panel) or ERK (lower panel). (B) A western blot of lysates of RAW264.7 cells, treated as indicated, was probed with antibodies that recognize phosphorylated ERK (upper panel), total ERK (middle panel) or COX-2 (lower panel).
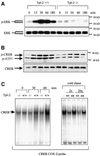
Fig. 3. LPS signals transduced via Tpl2 induce COX-2 transcription and enhance COX-2 mRNA stability. (A) RT–PCR was carried out on RNA derived from the indicated macrophage cultures, using primers specific for COX-2 or for the housekeeping gene HPRT. (B) COX-2 induction by LPS is regulated by Tpl2 at the level of transcription. (a) A nuclear run-on assay carried out using nuclei from Tpl2+/+ and Tpl2–/– macrophages harvested before and 2 h after stimulation with LPS. The experiment was repeated three times with similar results. (b) The mean value and SD of 32P incorporation measured in PhosphorImager units in one experiment carried out in triplicate. The induction of COX-2 transcription by LPS in Tpl2+/+ cells was statistically significant (p < 0.03). (C) Activation of the COX-2 promoter by LPS depends on signals transduced via Tpl2. (a) Schematic diagram of the COX-2 promoter. (b) Activity of a COX-2 promoter (–891 to +7)–luciferase reporter construct in RAW264.7 cells treated as indicated. RLVs were calculated as described in Materials and methods. Similar results were obtained in three independent experiments. (D) The stability of COX-2 mRNA is impaired in LPS-stimulated macrophages. COX-2 mRNA stability was measured as described in Materials and methods.
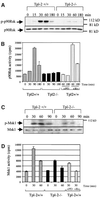
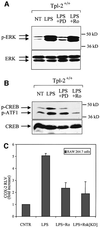
Fig. 4. Tpl2 regulates the phosphorylation and DNA binding activity of CREB in LPS-stimulated macrophages. (A) Kinetics of ERK phosphorylation in cell lysates from Tpl2+/+ and Tpl2–/– macrophages stimulated with LPS. (B) Kinetics of CREB/ATF1 phosphorylation in cell lysates from Tpl2+/+ and Tpl2–/– macrophages stimulated with LPS. (C) Induction of CREB DNA binding activity by LPS is Tpl2 dependent. Left panel: nuclear extracts of primary Tpl2+/+ and Tpl2–/– macrophages treated with LPS for 0, 30 or 60 min were analyzed by EMSA for binding to an oligonucleotide containing the CREB binding motif of the COX-2 promoter. Right panel: nuclear extracts from Tpl2+/+ cells treated with LPS for 1 h were incubated with excess unlabeled wild-type (wt) or mutant (mt) oligonucleotide probe prior to EMSA.
Fig. 5. Tpl2 transduces LPS signals leading to the phosphorylation and activation of the protein kinases p90Rsk and Msk1. (A) The phosphorylation of p90Rsk in LPS-stimulated Tpl2–/– macrophages is impaired. Lysates of LPS-stimulated macrophages were probed with antibodies to phosphorylated p90Rsk or to total p90Rsk. (B) p90Rsk activation by LPS in Tpl2–/– macrophages is impaired. Kinase activity is expressed in c.p.m. of [32P]ATP incorporated by p90Rsk immunoprecipitates. The data shown are mean values from triplicate determinations in a representative experiment. Four independent experiments gave similar results. PD is PD98059 and Ro is Ro318220. (C) The phosphorylation of Msk1 in LPS-stimulated Tpl2–/– macrophages is impaired. Lysates of LPS-stimulated macrophages were probed with antibodies to phosphorylated Msk1 or to total Msk1. (D) Msk1 activation by LPS in primary macrophages is Tpl2/ERK dependent. In vitro kinase assays were carried out on Msk1 immunoprecipitates using the synthetic peptide EILSRRPSYRK (CREBtide) as substrate. Kinase activity is expressed in c.p.m. of [32P]ATP incorporated in the substrate. The values shown are mean values from triplicate determinations in a representative experiment. Three independent experiments gave similar results.
Fig. 6. The phosphorylation of CREB and the activation of the COX-2 promoter by LPS depend on signals transduced by ERK, p90Rsk and Msk1. (A) Lysates of wild-type macrophages treated as indicated were probed with antibodies to phosphorylated ERK or total ERK. PD is PD58059 and Ro is Ro318220. (B) Both PD98059 and Ro318220 inhibit LPS-induced phosphorylation of CREB/ATF1 in Tpl2+/+ macrophages. Cell lysates from cultures identical to those described in (A) were probed with antibodies to phosphorylated CREB and ATF1 or to total CREB. (C) Both Msk1 and p90Rsk contribute to LPS-induced transactivation of the COX-2 promoter. The activity of a COX-2 promoter–luciferase reporter construct in RAW264.7 cells treated as indicated. Data are presented as the mean ± SD of the fold increase of the RLV in stimulated versus unstimulated control cultures.
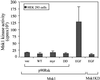
Fig. 7. P90Rsk does not regulate Msk1. HEK293 cells were transiently transfected with the indicated FLAG-tagged Msk1 constructs and with the indicated p90Rsk constructs. Control cells were stimulated with EGF. Anti-FLAG immunoprecipitates of lysates derived from these cultures were examined for Msk1 kinase activity. Mean values (± SD) from three independent experiments are shown.
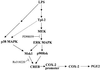
Fig. 8. Model of Tpl2-mediated COX-2 transactivation and PGE2 production in response to LPS. LPS engages a Tpl2-dependent pathway, which leads to the activation of ERK and p90RSK downstream of MEK. The same signals, in combination with p38 MAPK-transduced signals, activate Msk1. p90Rsk and Msk1, in turn, phosphorylate and activate CREB, which is critical for the transactivation of COX-2, a key enzyme for the biosynthesis of PGE2. The interrupted line connecting Tpl2 with p38MAPK indicates that non-obligatory signals transduced via Tpl2 may contribute to the activation of p38MAPK by LPS.
Similar articles
- The Tpl2 Kinase Regulates the COX-2/Prostaglandin E2 Axis in Adipocytes in Inflammatory Conditions.
Berthou F, Ceppo F, Dumas K, Massa F, Vergoni B, Alemany S, Cormont M, Tanti JF. Berthou F, et al. Mol Endocrinol. 2015 Jul;29(7):1025-36. doi: 10.1210/me.2015-1027. Epub 2015 May 28. Mol Endocrinol. 2015. PMID: 26020725 Free PMC article. - TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway.
Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. Dumitru CD, et al. Cell. 2000 Dec 22;103(7):1071-83. doi: 10.1016/s0092-8674(00)00210-5. Cell. 2000. PMID: 11163183 - Induction of cyclooxygenase-2 by heat shock protein 60 in macrophages and endothelial cells.
Billack B, Heck DE, Mariano TM, Gardner CR, Sur R, Laskin DL, Laskin JD. Billack B, et al. Am J Physiol Cell Physiol. 2002 Oct;283(4):C1267-77. doi: 10.1152/ajpcell.00609.2001. Am J Physiol Cell Physiol. 2002. PMID: 12225989 - Cot/tpl2 activity is required for TLR-induced activation of the Akt p70 S6k pathway in macrophages: Implications for NO synthase 2 expression.
López-Peláez M, Soria-Castro I, Boscá L, Fernández M, Alemany S. López-Peláez M, et al. Eur J Immunol. 2011 Jun;41(6):1733-41. doi: 10.1002/eji.201041101. Epub 2011 May 24. Eur J Immunol. 2011. PMID: 21469113 - Cot/Tpl2 regulates IL-23 p19 expression in LPS-stimulated macrophages through ERK activation.
Kakimoto K, Musikacharoen T, Chiba N, Bandow K, Ohnishi T, Matsuguchi T. Kakimoto K, et al. J Physiol Biochem. 2010 Mar;66(1):47-53. doi: 10.1007/s13105-010-0007-9. Epub 2010 Apr 20. J Physiol Biochem. 2010. PMID: 20405269
Cited by
- Eicosanoids in the innate immune response: TLR and non-TLR routes.
Alvarez Y, Valera I, Municio C, Hugo E, Padrón F, Blanco L, Rodríguez M, Fernández N, Crespo MS. Alvarez Y, et al. Mediators Inflamm. 2010;2010:201929. doi: 10.1155/2010/201929. Epub 2010 Jun 15. Mediators Inflamm. 2010. PMID: 20689730 Free PMC article. Review. - Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn's-like inflammatory bowel disease.
Kontoyiannis D, Boulougouris G, Manoloukos M, Armaka M, Apostolaki M, Pizarro T, Kotlyarov A, Forster I, Flavell R, Gaestel M, Tsichlis P, Cominelli F, Kollias G. Kontoyiannis D, et al. J Exp Med. 2002 Dec 16;196(12):1563-74. doi: 10.1084/jem.20020281. J Exp Med. 2002. PMID: 12486099 Free PMC article. - Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology.
Beinke S, Ley SC. Beinke S, et al. Biochem J. 2004 Sep 1;382(Pt 2):393-409. doi: 10.1042/BJ20040544. Biochem J. 2004. PMID: 15214841 Free PMC article. Review. - Phosphorylation of mitogen- and stress-activated protein kinase-1 in astrocytic inflammation: a possible role in inhibiting production of inflammatory cytokines.
Gong P, Xu X, Shi J, Ni L, Huang Q, Xia L, Nie D, Lu X, Chen J, Shi W. Gong P, et al. PLoS One. 2013 Dec 11;8(12):e81747. doi: 10.1371/journal.pone.0081747. eCollection 2013. PLoS One. 2013. PMID: 24349124 Free PMC article. - Effective Method for Accurate and Sensitive Quantitation of Rapid Changes of Newly Synthesized Proteins.
Tong M, Suttapitugsakul S, Wu R. Tong M, et al. Anal Chem. 2020 Jul 21;92(14):10048-10057. doi: 10.1021/acs.analchem.0c01823. Epub 2020 Jun 29. Anal Chem. 2020. PMID: 32531160 Free PMC article.
References
- Arthur J.S. and Cohen,P. (2000) MSK1 is required for CREB phosphorylation in response to mitogens in mouse embryonic stem cells. FEBS Lett., 482, 44–48. - PubMed
- Astiz M., Saha,D., Lustbader,D., Lin,R. and Rackow,E. (1996) Monocyte response to bacterial toxins, expression of cell surface receptors, and release of anti-inflammatory cytokines during sepsis. J. Lab. Clin. Med., 128, 594–600. - PubMed
- Barrios-Rodiles M., Tiraloche,G. and Chadee,K. (1999) Lipopoly saccharide modulates cyclooxygenase-2 transcriptionally and posttranscriptionally in human macrophages independently from endogenous IL-1β and TNF-α. J. Immunol., 163, 963–969. - PubMed
- Caivano M. and Cohen,P. (2000) Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1β in RAW264 macrophages. J. Immunol., 164, 3018–3025. - PubMed
- Ceci J.D., Patriotis,C.P., Tsatsanis,C., Makris,A.M., Kovatch,R., Swing,D.A., Jenkins,N.A., Tsichlis,P.N. and Copeland,N.G. (1997) Tpl-2 is an oncogenic kinase that is activated by carboxy-terminal truncation. Genes Dev., 11, 688–700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous