IkappaB kinase signaling is essential for maintenance of mature B cells - PubMed (original) (raw)
IkappaB kinase signaling is essential for maintenance of mature B cells
Manolis Pasparakis et al. J Exp Med. 2002.
Abstract
Nuclear factor (NF)-kappaB proteins play crucial roles in immune responses and cellular survival. Activation of NF-kappaB is mediated by the IkappaB kinase (IKK) complex, which is composed of two kinases, IKK1 and IKK2, and a regulatory subunit termed NF-kappaB essential modulator (NEMO). IKK2- and NEMO-deficient mice die at early embryonic stages. We therefore used conditional gene targeting to evaluate the role of these proteins in B cells in adult mice. B lineage-specific disruption of either IKK signaling by deletion of NEMO, or of IKK2-specific signals by ablation of IKK2 activity leads to the disappearance of mature B lymphocytes. We conclude that maintenance of mature B cells depends on IKK-mediated activation of NF-kappaB.
Figures
Figure 1.
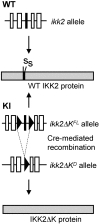
The conditional ikk2_Δ_K allele. Schematic representation of the WT ikk2 allele, which produces WT IKK2 protein and the ikk2_Δ_K allele, which after deletion of loxP-flanked exon 7 generates a kinase-dead version of IKK2 (IKK2ΔK). Open and filled boxes represent exons, triangles represent loxP sites, and the two serines of the IKK2 activation loop are indicated using the amino acid single letter code.
Figure 2.
FACS® analysis of BM B cell populations. (A) CD19-Cre/Ikk2 FL/D, Ikk2Δ_K_ FL/D, and controls and (B) CD19-Cre/Nemo FL/Y and control mice. Genotypes are as indicated. Cell surface markers are shown as coordinates and gated cell populations are indicated in brackets. The numbers next to boxed lymphocyte populations refer to the percentages of live cells in the lymphocyte gate.
Figure 3.
FACS® analysis of splenic B cell populations. (A) CD19-Cre/Ikk2 FL/D, Ikk2ΔK FL/D, and controls and (B) CD19-Cre/Nemo FL/Y and control mice. Genotypes are as indicated. Cell surface markers are shown as coordinates and gated cell populations are indicated in brackets. The numbers next to boxed lymphocyte populations refer to the percentages of live cells in the lymphocyte gate.
Figure 4.
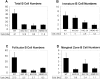
Reduction of B cell numbers in _CD19Cre/IKK-_conditional mice. Absolute numbers of B cell subpopulations in the spleens of CD19-Cre/Ikk2 FL/D, CD19-Cre/Ikk2_Δ_K FL/D, and CD19-Cre/Nemo FL/Y compared with control mice are shown. (A) Total B cell numbers, (B) IM B cells, (C) follicular B cells, and (D) marginal zone B cells. Bar charts showing the absolute cell numbers for the indicated B cell population in the spleen for each of the following mouse strains: CT (controls), IKK2 KO (CD19-Cre/Ikk2 FL/D), IKK2 DK (CD19-Cre/Ikk2_Δ_K FL/D), and NEMO KO (CD19-Cre/Nemo FL/Y). For each group three to eight mice were analyzed. Error bars indicate standard deviation. The absolute cell numbers for each population were calculated by multiplying the percentage of each B cell subset of all live splenocytes obtained from the FACS® analysis with the total number of live cells recovered from the perspective spleen.
Figure 5.
Counterselection against Ikk D/D, Ikk2_Δ_K D/D, and Nemo D/Y B cells in the spleen. Southern blot analyses of sorted splenic B cell populations of mice of the indicated genotypes are shown. (A) CD19-Cre/Ikk2 FL/D mice, (B) CD19-Cre/Ikk2_Δ_K FL/D mice, (C) CD19-Cre/Nemo FL/Y mice, (D) CD19-Cre/Ikk2_Δ_K FL/WT mice. T, tail; IgM− (CD19+B220+IgM−: pro- and pre-B cells); IgM+ (CD19+B220+IgM+ B cells); IM (CD19+ CD21lowHSAhigh: immature B cells); FO (CD19+CD21intHSA−: follicular B cells); MZ (CD19+CD21highHSAint: marginal zone B cells). The percentage of deleted alleles and cells of the deleted genotype is given below the blots. Each blot is a representative example of at least four mice analyzed. Del (%), percent of deleted alleles.
Figure 6.
Increased B cell turnover in the spleens of CD19-Cre/Ikk2_Δ_K FL/D mice compared with control mice. Analysis of BrdU incorporation by splenic B cells of mice of the indicated genotypes after 1 wk of BrdU administration in the drinking water. Numbers indicate percentages of BrdU-positive and BrdU-negative B cells.
Figure 7.
Block of B cell influx from the BM in CD19-Cre/Ikk2_Δ_K FL/D mice leads to disappearance of B cells of the deleted genotype in the spleen. (A) Verification of the block in B cell development after injection of anti–IL-7R antibodies by FACS® analysis. Noninjected control mice are compared with mice that had received injections of anti–IL-7R antibodies for 4 wk. The genotypes are as indicated above and cell surface markers are shown as coordinates and gated cell populations are indicated in brackets. The numbers next to boxed lymphocyte populations refer to the percentages of live cells in the lymphocyte gate. (B) Southern blot analysis of Cre mediated deletion in total splenic B cells from two different (#1, #2) CD19-Cre/Ikk2_Δ_K FL/D mice. The results are representative of six different mice. (C) Southern blot analysis of Cre mediated deletion in total splenic B cells from three different (#1–3) CD19-Cre/Ikk2_Δ_K FL/D mice that received anti–IL-7R antibody injections for 4 wk.
Similar articles
- Mature T cells depend on signaling through the IKK complex.
Schmidt-Supprian M, Courtois G, Tian J, Coyle AJ, Israël A, Rajewsky K, Pasparakis M. Schmidt-Supprian M, et al. Immunity. 2003 Sep;19(3):377-89. doi: 10.1016/s1074-7613(03)00237-1. Immunity. 2003. PMID: 14499113 - Molecular genetic analysis of human herpes virus 8-encoded viral FLICE inhibitory protein-induced NF-kappaB activation.
Matta H, Sun Q, Moses G, Chaudhary PM. Matta H, et al. J Biol Chem. 2003 Dec 26;278(52):52406-11. doi: 10.1074/jbc.M307308200. Epub 2003 Oct 15. J Biol Chem. 2003. PMID: 14561765 - Regulation and function of IKK and IKK-related kinases.
Häcker H, Karin M. Häcker H, et al. Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review. - Zebrafish IkappaB kinase 1 negatively regulates NF-kappaB activity.
Correa RG, Matsui T, Tergaonkar V, Rodriguez-Esteban C, Izpisua-Belmonte JC, Verma IM. Correa RG, et al. Curr Biol. 2005 Jul 26;15(14):1291-5. doi: 10.1016/j.cub.2005.06.023. Curr Biol. 2005. PMID: 16051172 - NF-kappa B defects in humans: the NEMO/incontinentia pigmenti connection.
Courtois G, Israël A. Courtois G, et al. Sci STKE. 2000 Nov 14;2000(58):pe1. doi: 10.1126/stke.2000.58.pe1. Sci STKE. 2000. PMID: 11752619 Review.
Cited by
- Testing gene function early in the B cell lineage in mb1-cre mice.
Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Hobeika E, et al. Proc Natl Acad Sci U S A. 2006 Sep 12;103(37):13789-94. doi: 10.1073/pnas.0605944103. Epub 2006 Aug 29. Proc Natl Acad Sci U S A. 2006. PMID: 16940357 Free PMC article. - The role of nuclear factor-kappaB essential modulator (NEMO) in B cell development and survival.
Kim S, La Motte-Mohs RN, Rudolph D, Zuniga-Pflucker JC, Mak TW. Kim S, et al. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1203-8. doi: 10.1073/pnas.0337707100. Epub 2003 Jan 21. Proc Natl Acad Sci U S A. 2003. PMID: 12538858 Free PMC article. - B-cell survival and development controlled by the coordination of NF-κB family members RelB and cRel.
Almaden JV, Liu YC, Yang E, Otero DC, Birnbaum H, Davis-Turak J, Asagiri M, David M, Goldrath AW, Hoffmann A. Almaden JV, et al. Blood. 2016 Mar 10;127(10):1276-86. doi: 10.1182/blood-2014-10-606988. Epub 2016 Jan 14. Blood. 2016. PMID: 26773039 Free PMC article. - BAFF and the plasticity of peripheral B cell tolerance.
Stadanlick JE, Cancro MP. Stadanlick JE, et al. Curr Opin Immunol. 2008 Apr;20(2):158-61. doi: 10.1016/j.coi.2008.03.015. Epub 2008 May 2. Curr Opin Immunol. 2008. PMID: 18456486 Free PMC article. Review. - Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation.
Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, Ploegh HL, Coyle AJ, Rajewsky K. Schmidt-Supprian M, et al. Proc Natl Acad Sci U S A. 2004 Mar 30;101(13):4566-71. doi: 10.1073/pnas.0400885101. Epub 2004 Mar 16. Proc Natl Acad Sci U S A. 2004. PMID: 15070758 Free PMC article.
References
- Ghosh, S., M.J. May, and E.B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225–260. - PubMed
- Solan, N.J., H. Miyoshi, E.M. Carmona, G.D. Bren, and C.V. Paya. 2002. RelB cellular regulation and transcriptional activity are regulated by p100. J. Biol. Chem. 277:1405–1418. - PubMed
- Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu. Rev. Immunol. 18:621–663. - PubMed
- Israel, A. 2000. The IKK complex: an integrator of all signals that activate NF-kappaB? Trends Cell Biol. 10:129–133. - PubMed
- Rothwarf, D.M., and M. Karin. 1999. The NF-κB activation pathway: a paradigm in information transfer from membrane to nucleus. Sci. STKE. 1999:RE1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous