Interleukin 2 signaling is required for CD4(+) regulatory T cell function - PubMed (original) (raw)
Interleukin 2 signaling is required for CD4(+) regulatory T cell function
Gláucia C Furtado et al. J Exp Med. 2002.
Abstract
Mice deficient in interleukin (IL)-2 production or the IL-2 receptor alpha or beta chains develop a lethal autoimmune syndrome. CD4(+) regulatory T cells have been shown to prevent autoimmune diseases, allograft rejection, and to down-regulate antibody responses against foreign antigens. To assess the role of IL-2 in the generation and function of regulatory T cells, we transferred CD4(+) T cells from mice genetically deficient in IL-2 or IL-2R(alpha) (CD25) expression. A small number of splenic or thymic CD4(+) T cells from IL-2 knockout mice can protect mice from spontaneous experimental autoimmune encephalomyelitis (EAE). In contrast, splenic or thymic CD4(+) T cells from CD25 knockout donor mice conferred little or no protection. We conclude that T cells with regulatory potential can develop, undergo thymic selection, and migrate to the peripheral lymphoid organs in the absence of IL-2, and do not protect from disease by means of IL-2 secretion. However, IL-2 signaling in regulatory T cells is essential for their protective function. Altogether, our results favor a model whereby IL-2 induces regulatory T cell activity.
Figures
Figure 1.
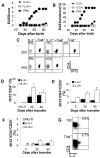
CD4+ splenocytes from IL-2 KO mice efficiently protect against spontaneous EAE. (A–G) 23–27-d-old T/α−β− IL-2+ mice were injected with PBS (n = 6) or with 3 × 105 purified CD4+ T cells from spleens of 3–4-wk-old IL-2−/− (n = 10) or IL-2+/− mice (n = 7). Mice were observed for 90 d and EAE was scored as described in Materials and Methods. (A) Average clinical EAE score. (B) Average EAE incidence. (C–G) Peripheral blood lymphocytes were collected from all recipient mice 20 and 40 d after cell transfer, stained with anti-MBP TCR (3H12) FITC, CD25 PE, CD4 PerCP, and CD3 APC and analyzed by FACS®. (C) Representative staining indicating the identification of donor-derived (3H12−) and recipient-generated (3H12+) T cells. Gated on CD3+ cells. (D) Expansion of donor-derived (3H12−) T cells. Cells were stained and gated as in C. Shown are the mean percentages of clonotype-negative cells from all mice in each group ± SD. (E) Average percentage of CD25 positive T cells (± SD) among the donor-derived CD4+ cells (CD4+3H12_−_ gate). (F) Average percentage of CD25-positive T cells (± SD) among the recipient-generated CD4+ cells (CD4+3H12+ gate). (G) IL-2 production by MBP-specific T cells. Intracellular IL-2 staining of deep cervical lymph node cells from a healthy 7-wk-old T/R+ and a 7-wk-old T/α−β− mouse with early clinical signs of spontaneous EAE. Surface staining with anti-CD4 and 3H12. Gated on 3H12+ cells.
Figure 2.
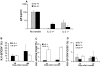
IL-2 KO cells prevent hyper IgE response. 17/9 DO11.10 RAG−/− BALB/c mice were transferred with 1.6 × 107 spleen cells from 3-wk-old IL-2+/− or IL-2−/− BALB/c donor mice. The recipient mice were immunized by intraperitoneal route with OVA-HA in alum 24 h after the transfer of cells. n = 3 recipient mice per group. Data representative of one of two experiments performed (A). Serum IgE levels 19 and 44 d after immunization. Data presented as mean IgE concentration ± SD (B–D). Peripheral blood lymphocytes were stained with KJ1–26 FITC, CD25 PE, and CD4 PerCP and analyzed by FACS®. Data presented as mean percentage ± SD. (B) Expansion of CD4+ donor-derived cells (CD4+KJ1–26−) as a percentage of total lymphocytes (forward scatter × side scatter lymphocyte gate). (C) Average percentage of CD25 positive T cells (± SD) among the donor-derived CD4+ cells (CD4+KJ1–26− gate). (D) Average percentage of CD25 positive T cells (± SD) among the recipient's CD4+ cells (CD4+KJ1–26+ gate).
Figure 3.
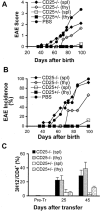
CD25 KO cells do not protect efficiently against spontaneous EAE. 23–27 d-old T/α−β− mice were injected with PBS (n = 8) or with 3 × 105 CD4+ T cells from spleen (spl) of CD25− / − mice (n = 13), spleen of CD25+/− mice (n = 8), thymus (thy) of CD25− / − mice (n = 3), or thymus of CD25+/− mice (n = 3) purified from 3–4-wk-old CD25−/− and CD25+/− donor mice. Mice were observed for 100 d and EAE were scored as described in Materials and Methods. (A) Average clinical EAE score. (B) Average EAE incidence. (C) The expansion of donor-derived (3H12−) CD4+ T cells in each experimental group was assessed by peripheral blood staining 25 and 45 d after cell transfer (CD4+ lymphocyte gate). Data presented as mean percentage ± SD.
Figure 4.
Model depicting the possible effect of IL-2 on regulatory T cells based on the spontaneous EAE data. (reference 1). Self-specific Th precursor cells encounter self antigen and (reference 2) secrete IL-2. Although costimulatory molecules are not drawn in the model due to lack of space, in the spontaneous EAE system the generation of MBP-specific Th effector cells is CD28-dependent (reference 34). (1') Regulatory T cells or their precursors interact via TCR with yet unknown ligands, and (2') up-regulate the IL-2 receptor. (3') IL-2 promotes regulatory T cell differentiation or expansion in the periphery, and/or (4') triggers regulatory functions such as secretion of down-modulatory cytokines. In this scheme, the source of IL-2 is the self-specific T cell population, although other activated host cell types could also produce IL-2 (reference 35).
Similar articles
- CD4(+) T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti-myelin basic protein T cell receptor transgenic mice.
Van de Keere F, Tonegawa S. Van de Keere F, et al. J Exp Med. 1998 Nov 16;188(10):1875-82. doi: 10.1084/jem.188.10.1875. J Exp Med. 1998. PMID: 9815265 Free PMC article. - Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers.
Almeida AR, Legrand N, Papiernik M, Freitas AA. Almeida AR, et al. J Immunol. 2002 Nov 1;169(9):4850-60. doi: 10.4049/jimmunol.169.9.4850. J Immunol. 2002. PMID: 12391195 - Endogenous CD4+BV8S2- T cells from TG BV8S2+ donors confer complete protection against spontaneous experimental encephalomyelitis (Sp-EAE) in TCR transgenic, RAG-/- mice.
Matejuk A, Buenafe AC, Dwyer J, Ito A, Silverman M, Zamora A, Subramanian S, Vandenbark AA, Offner H. Matejuk A, et al. J Neurosci Res. 2003 Jan 1;71(1):89-103. doi: 10.1002/jnr.10450. J Neurosci Res. 2003. PMID: 12478617 - The main function of IL-2 is to promote the development of T regulatory cells.
Malek TR. Malek TR. J Leukoc Biol. 2003 Dec;74(6):961-5. doi: 10.1189/jlb.0603272. Epub 2003 Sep 2. J Leukoc Biol. 2003. PMID: 12960253 Review. - Regulatory T cells in spontaneous autoimmune encephalomyelitis.
Furtado GC, Olivares-Villagómez D, Curotto de Lafaille MA, Wensky AK, Latkowski JA, Lafaille JJ. Furtado GC, et al. Immunol Rev. 2001 Aug;182:122-34. doi: 10.1034/j.1600-065x.2001.1820110.x. Immunol Rev. 2001. PMID: 11722629 Review.
Cited by
- Updates in chronic graft-versus-host disease: novel treatments and best practices in the current era.
Vadakkel G, Eng S, Proli A, Ponce DM. Vadakkel G, et al. Bone Marrow Transplant. 2024 Oct;59(10):1360-1368. doi: 10.1038/s41409-024-02370-8. Epub 2024 Jul 31. Bone Marrow Transplant. 2024. PMID: 39080470 Review. - The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease.
Cooke KR, Luznik L, Sarantopoulos S, Hakim FT, Jagasia M, Fowler DH, van den Brink MRM, Hansen JA, Parkman R, Miklos DB, Martin PJ, Paczesny S, Vogelsang G, Pavletic S, Ritz J, Schultz KR, Blazar BR. Cooke KR, et al. Biol Blood Marrow Transplant. 2017 Feb;23(2):211-234. doi: 10.1016/j.bbmt.2016.09.023. Epub 2016 Oct 3. Biol Blood Marrow Transplant. 2017. PMID: 27713092 Free PMC article. Review. - Allopeptides and the alloimmune response.
Bharat A, Mohanakumar T. Bharat A, et al. Cell Immunol. 2007 Jul;248(1):31-43. doi: 10.1016/j.cellimm.2007.03.010. Cell Immunol. 2007. PMID: 18023633 Free PMC article. Review. - Interleukin-2 receptor signaling: at the interface between tolerance and immunity.
Malek TR, Castro I. Malek TR, et al. Immunity. 2010 Aug 27;33(2):153-65. doi: 10.1016/j.immuni.2010.08.004. Immunity. 2010. PMID: 20732639 Free PMC article. Review. - Cellular Immune Responses in Islet Xenograft Rejection.
Hu M, Hawthorne WJ, Yi S, O'Connell PJ. Hu M, et al. Front Immunol. 2022 Jul 7;13:893985. doi: 10.3389/fimmu.2022.893985. eCollection 2022. Front Immunol. 2022. PMID: 35874735 Free PMC article. Review.
References
- Sadlack, B., H. Merz, H. Schorle, A. Schimpl, A.C. Feller, and I. Horak. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 75:253–261. - PubMed
- Sadlack, B., J. Lohler, H. Schorle, G. Klebb, H. Haber, E. Sickel, R.J. Noelle, and I. Horak. 1995. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur. J. Immunol. 25:3053–3059. - PubMed
- Willerford, D.M., J. Chen, J.A. Ferry, L. Davidson, A. Ma, and F.W. Alt. 1995. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 3:521–530. - PubMed
- Suzuki, H., T.M. Kundig, C. Furlonger, A. Wakeham, E. Timms, T. Matsuyama, R. Schmits, J.J. Simard, P.S. Ohashi, H. Griesser, et al. 1995. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 268:1472–1476. - PubMed
- Lenardo, M.J. 1991. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 353:858–861. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous