Intrinsic and extrinsic contributions to stochasticity in gene expression - PubMed (original) (raw)
Intrinsic and extrinsic contributions to stochasticity in gene expression
Peter S Swain et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Gene expression is a stochastic, or "noisy," process. This noise comes about in two ways. The inherent stochasticity of biochemical processes such as transcription and translation generates "intrinsic" noise. In addition, fluctuations in the amounts or states of other cellular components lead indirectly to variation in the expression of a particular gene and thus represent "extrinsic" noise. Here, we show how the total variation in the level of expression of a given gene can be decomposed into its intrinsic and extrinsic components. We demonstrate theoretically that simultaneous measurement of two identical genes per cell enables discrimination of these two types of noise. Analytic expressions for intrinsic noise are given for a model that involves all the major steps in transcription and translation. These expressions give the sensitivity to various parameters, quantify the deviation from Poisson statistics, and provide a way of fitting experiment. Transcription dominates the intrinsic noise when the average number of proteins made per mRNA transcript is greater than approximately 2. Below this number, translational effects also become important. Gene replication and cell division, included in the model, cause protein numbers to tend to a limit cycle. We calculate a general form for the extrinsic noise and illustrate it with the particular case of a single fluctuating extrinsic variable-a repressor protein, which acts on the gene of interest. All results are confirmed by stochastic simulation using plausible parameters for Escherichia coli.
Figures
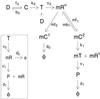
Figure 1
Reaction scheme detailing constitutive expression of a protein P. All molecular species shown are intrinsic variables. Transcription is modeled (15) as reversible binding of RNAP to promoter, D (rates _f_0 and _b_0). Isomerization from closed to open complex and initiation of transcription are approximated as a first-order process (rate _k_0). Only the leader region of the mRNA, mR U, is followed. It is made by transcribing polymerase, T, at rate ν0. mRNA is degraded by the binding of the degradosome (rate _mf_0) to form complex _mC_1, which decays in a first-order manner. Following ref. , ribosomes compete with degradosomes for the leader region of the mRNA and bind reversibly (rates _mf_1 and _mb_1). Start of translation is from the _mC_2 state with rate _k_1, which frees mR U for further binding. Protein is translated (rate ν1) in the mT state and decays with rate _d_1. Inset shows a simplified model of translation, with mR now designating an entire mRNA molecule, degrading at rate _d_′0, and is translated with rate ν′1.
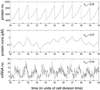
Figure 2
Simulation results for protein and mRNA number using the model of Fig. 1 Inset. A strong promoter is used (k_0 = 0.1 s−1), and there is just one copy of the gene on the chromosome. On average, 15 proteins are synthesized per mRNA transcript, and the mRNA half-life is 1 min. Gene replication occurs every t d = 0.4_T into the cell cycle and is marked with a small dot on the time axis. All other parameters are given in the supporting information. The total noise ηtot is defined in Eqs. 6 and 7, with the overbar, in this simple case, just denoting a time (cell cycle) average. This is given for each species in the upper right-hand corner.
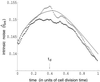
Figure 3
Comparison of analytic solution and stochastic simulation. The noise in protein level (averaged over 5,000 runs) for the three different models is plotted as a function of time (in units of the cell cycle). Upper light dotted curve is the result of a simulation of the full model of protein expression, Fig. 1, with _k_0 = 0.01 s−1. Dotted curve is a simulation of Fig. 1 Inset, whereas the full curve is a plot of Eq. 17. At the beginning of the cell cycle, η̂int is slightly greater than that at the end because of the random partitioning of proteins and mRNA into daughter cells on division.
Similar articles
- Quantifying intrinsic and extrinsic variability in stochastic gene expression models.
Singh A, Soltani M. Singh A, et al. PLoS One. 2013 Dec 31;8(12):e84301. doi: 10.1371/journal.pone.0084301. eCollection 2013. PLoS One. 2013. PMID: 24391934 Free PMC article. - Stochasticity in single gene expression with both intrinsic noise and fluctuation in kinetic parameters.
Lei J. Lei J. J Theor Biol. 2009 Feb 21;256(4):485-92. doi: 10.1016/j.jtbi.2008.10.028. Epub 2008 Nov 12. J Theor Biol. 2009. PMID: 19056400 - Intercellular Variability in Protein Levels from Stochastic Expression and Noisy Cell Cycle Processes.
Soltani M, Vargas-Garcia CA, Antunes D, Singh A. Soltani M, et al. PLoS Comput Biol. 2016 Aug 18;12(8):e1004972. doi: 10.1371/journal.pcbi.1004972. eCollection 2016 Aug. PLoS Comput Biol. 2016. PMID: 27536771 Free PMC article. - Modeling cell-to-cell stochastic variability in intrinsic apoptosis pathway.
Ooi HK, Ma L. Ooi HK, et al. Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:5498-501. doi: 10.1109/EMBC.2012.6347239. Annu Int Conf IEEE Eng Med Biol Soc. 2012. PMID: 23367174 Review. - Noise in bacterial gene expression.
Engl C. Engl C. Biochem Soc Trans. 2019 Feb 28;47(1):209-217. doi: 10.1042/BST20180500. Epub 2018 Dec 21. Biochem Soc Trans. 2019. PMID: 30578346 Review.
Cited by
- A synthetic biology approach to understanding cellular information processing.
Riccione KA, Smith RP, Lee AJ, You L. Riccione KA, et al. ACS Synth Biol. 2012 Sep 21;1(9):389-402. doi: 10.1021/sb300044r. ACS Synth Biol. 2012. PMID: 23411668 Free PMC article. Review. - Genetic Redundancies Enhance Information Transfer in Noisy Regulatory Circuits.
Rodrigo G, Poyatos JF. Rodrigo G, et al. PLoS Comput Biol. 2016 Oct 14;12(10):e1005156. doi: 10.1371/journal.pcbi.1005156. eCollection 2016 Oct. PLoS Comput Biol. 2016. PMID: 27741249 Free PMC article. - Cellular noise regulons underlie fluctuations in Saccharomyces cerevisiae.
Stewart-Ornstein J, Weissman JS, El-Samad H. Stewart-Ornstein J, et al. Mol Cell. 2012 Feb 24;45(4):483-93. doi: 10.1016/j.molcel.2011.11.035. Mol Cell. 2012. PMID: 22365828 Free PMC article. - Understanding the Principles of Pattern Formation Driven by Notch Signaling by Integrating Experiments and Theoretical Models.
Bocci F, Onuchic JN, Jolly MK. Bocci F, et al. Front Physiol. 2020 Jul 31;11:929. doi: 10.3389/fphys.2020.00929. eCollection 2020. Front Physiol. 2020. PMID: 32848867 Free PMC article. Review. - Cell cycle control by a minimal Cdk network.
Gérard C, Tyson JJ, Coudreuse D, Novák B. Gérard C, et al. PLoS Comput Biol. 2015 Feb 6;11(2):e1004056. doi: 10.1371/journal.pcbi.1004056. eCollection 2015 Feb. PLoS Comput Biol. 2015. PMID: 25658582 Free PMC article.
References
- Guptasarma P. BioEssays. 1995;17:987–997. - PubMed
- Spudich J L, Koshland D E., Jr Nature (London) 1976;262:467–471. - PubMed
- McAdams H H, Arkin A. Trends Genet. 1999;15:65–69. - PubMed
- Ko M S H. J Theor Biol. 1991;153:181–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources