Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol - PubMed (original) (raw)
Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol
Zhengfan Jiang et al. Mol Cell Biol. 2002 Oct.
Abstract
Interleukin-1 (IL-1) receptor-associated kinase (IRAK) plays an important role in the sequential formation and activation of IL-1-induced signaling complexes. Previous studies showed that IRAK is recruited to the IL-1-receptor complex, where it is hyperphosphorylated. We now find that the phosphorylated IRAK in turn recruits TRAF6 to the receptor complex (complex I), which differs from the previous concept that IRAK interacts with TRAF6 after it leaves the receptor. IRAK then brings TRAF6 to TAK1, TAB1, and TAB2, which are preassociated on the membrane before stimulation to form the membrane-associated complex II. The formation of complex II leads to the phosphorylation of TAK1 and TAB2 on the membrane by an unknown kinase, followed by the dissociation of TRAF6-TAK1-TAB1-TAB2 (complex III) from IRAK and consequent translocation of complex III to the cytosol. The formation of complex III and its interaction with additional cytosolic factors lead to the activation of TAK1, resulting in NF-kappaB and JNK activation. Phosphorylated IRAK remains on the membrane and eventually is ubiquitinated and degraded. Taken together, the new data reveal that IRAK plays a critical role in mediating the association and dissociation of IL-1-induced signaling complexes, functioning as an organizer and transporter in IL-1-dependent signaling.
Figures
FIG. 1.
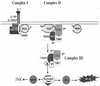
A revised model for IL-1-dependent signaling. Upon IL-1 stimulation, the adaptor molecules MyD88 and Tollip are recruited to the IL-1 receptor complex, which then recruits IRAK. IRAK is hyperphosphorylated, mediating the recruitment of TRAF6 to the receptor complex (complex I). IRAK4 is included in complex I, since it has been suggested that it may function as an IRAK kinase. IRAK-TRAF6 then leaves complex I to interact with preassociated TAK1, TAB1, and TAB2 on the membrane, resulting in the formation of complex II. The formation of complex II leads to the phosphorylation of TAK1 and TAB2, which facilitates the formation and translocation of complex III from the membrane to the cytosol. The formation of complex III and its interaction with additional factors in the cytosol lead to the activation of TAK1. The activated TAK1 causes, directly or indirectly, the activation of IKK and MKK6, resulting in activation of NF-κB and JNK. X and Y are unknown components. P*, phosphorylation; U, ubiquitination.
FIG. 2.
Formation of complex I. (A) Recruitment of TRAF6 to the IL-1 receptor. Extracts of 293 cells either untreated or treated with IL-1 for the indicated times, immunoprecipitated (IP) with anti-IL-1R or anti-IRAK antibody, and subjected to Western blot analysis with anti-IL-1R, anti-IRAK, or anti-TRAF6 antibody. (B) The recruitment of TRAF6 to IL-1 receptor is IRAK dependent. Extracts of 293 cells (wild type [WT]), IRAK-deficient cells (I1A), I1A cells stably transfected with IRAK (I1A-IRAK), either untreated or treated with IL-1 for 10 min were immunoprecipitated with anti-IL-1R antibody and subjected to Western blot analysis with anti-TRAF6 and anti-IL-1R antibodies. (C) Domains of IRAK required for the recruitment of IRAK-TRAF6 to IL-1 receptor. Extracts of 293 cells (WT), IRAK-deficient I1A cells transfected with IRAK deletion mutants dDD, dUD, dKD, dC2, dC1C2, and DD+UD+C1 either untreated or treated with IL-1 for 10 min were immunoprecipitated with anti-IL-1R antibody and probed with anti-IRAK, anti-TRAF6, or anti-IL-1R antibody.
FIG. 2.
Formation of complex I. (A) Recruitment of TRAF6 to the IL-1 receptor. Extracts of 293 cells either untreated or treated with IL-1 for the indicated times, immunoprecipitated (IP) with anti-IL-1R or anti-IRAK antibody, and subjected to Western blot analysis with anti-IL-1R, anti-IRAK, or anti-TRAF6 antibody. (B) The recruitment of TRAF6 to IL-1 receptor is IRAK dependent. Extracts of 293 cells (wild type [WT]), IRAK-deficient cells (I1A), I1A cells stably transfected with IRAK (I1A-IRAK), either untreated or treated with IL-1 for 10 min were immunoprecipitated with anti-IL-1R antibody and subjected to Western blot analysis with anti-TRAF6 and anti-IL-1R antibodies. (C) Domains of IRAK required for the recruitment of IRAK-TRAF6 to IL-1 receptor. Extracts of 293 cells (WT), IRAK-deficient I1A cells transfected with IRAK deletion mutants dDD, dUD, dKD, dC2, dC1C2, and DD+UD+C1 either untreated or treated with IL-1 for 10 min were immunoprecipitated with anti-IL-1R antibody and probed with anti-IRAK, anti-TRAF6, or anti-IL-1R antibody.
FIG. 2.
Formation of complex I. (A) Recruitment of TRAF6 to the IL-1 receptor. Extracts of 293 cells either untreated or treated with IL-1 for the indicated times, immunoprecipitated (IP) with anti-IL-1R or anti-IRAK antibody, and subjected to Western blot analysis with anti-IL-1R, anti-IRAK, or anti-TRAF6 antibody. (B) The recruitment of TRAF6 to IL-1 receptor is IRAK dependent. Extracts of 293 cells (wild type [WT]), IRAK-deficient cells (I1A), I1A cells stably transfected with IRAK (I1A-IRAK), either untreated or treated with IL-1 for 10 min were immunoprecipitated with anti-IL-1R antibody and subjected to Western blot analysis with anti-TRAF6 and anti-IL-1R antibodies. (C) Domains of IRAK required for the recruitment of IRAK-TRAF6 to IL-1 receptor. Extracts of 293 cells (WT), IRAK-deficient I1A cells transfected with IRAK deletion mutants dDD, dUD, dKD, dC2, dC1C2, and DD+UD+C1 either untreated or treated with IL-1 for 10 min were immunoprecipitated with anti-IL-1R antibody and probed with anti-IRAK, anti-TRAF6, or anti-IL-1R antibody.
FIG. 3.
IRAK-mediated interactions between signaling components. IL-1-induced interactions between signaling molecules were examined by coimmunoprecipitation in IRAK-deficient I1A cells transfected with different IRAK deletion constructs. DD, death domain; UD, domain of unknown function, KD, kinase domain; dDD, deletion of death domain; dUD, deletion of undetermined domain; dKD, deletion of kinase domain; dC, deletion of C-terminal domain.
FIG. 4.
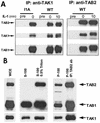
Interaction of IRAK-TRAF6 with TAK1 and TAB2. (A) Kinetics. Extracts of 293 cells, either untreated or treated with IL-1, were immunoprecipitated (IP) with anti-TAK1 or anti-TAB2 antibody and subjected to Western blot analyses with anti-IRAK, anti-TRAF6, anti-TAK1, and anti-TAB2 antibodies. (B) The interaction between TRAF6 and TAB2 is IRAK dependent. Extracts of 293 cells (wild type [WT]), IRAK-deficient cells (I1A), and I1A cells stably transfected with IRAK (I1A-IRAK), either untreated or treated with IL-1 for 10 min were immunoprecipitated with anti-TAB2 antibody and probed with anti-TRAF6 antibody. (C) Domains of IRAK required for the interaction of IRAK-TRAF6 with TAK1. Extracts of 293 cells (WT) and IRAK-deficient cells I1A, stably transfected with different IRAK deletion mutants (dDD, dUD, dKD, dC2, dC1C2, and DD+UD+C1), either untreated or treated with IL-1 for 10 min, were immunoprecipitated with anti-TAK1 antibody and probed with anti-IRAK, anti-TRAF6, and anti-TAK1 antibodies.
FIG. 4.
Interaction of IRAK-TRAF6 with TAK1 and TAB2. (A) Kinetics. Extracts of 293 cells, either untreated or treated with IL-1, were immunoprecipitated (IP) with anti-TAK1 or anti-TAB2 antibody and subjected to Western blot analyses with anti-IRAK, anti-TRAF6, anti-TAK1, and anti-TAB2 antibodies. (B) The interaction between TRAF6 and TAB2 is IRAK dependent. Extracts of 293 cells (wild type [WT]), IRAK-deficient cells (I1A), and I1A cells stably transfected with IRAK (I1A-IRAK), either untreated or treated with IL-1 for 10 min were immunoprecipitated with anti-TAB2 antibody and probed with anti-TRAF6 antibody. (C) Domains of IRAK required for the interaction of IRAK-TRAF6 with TAK1. Extracts of 293 cells (WT) and IRAK-deficient cells I1A, stably transfected with different IRAK deletion mutants (dDD, dUD, dKD, dC2, dC1C2, and DD+UD+C1), either untreated or treated with IL-1 for 10 min, were immunoprecipitated with anti-TAK1 antibody and probed with anti-IRAK, anti-TRAF6, and anti-TAK1 antibodies.
FIG. 4.
Interaction of IRAK-TRAF6 with TAK1 and TAB2. (A) Kinetics. Extracts of 293 cells, either untreated or treated with IL-1, were immunoprecipitated (IP) with anti-TAK1 or anti-TAB2 antibody and subjected to Western blot analyses with anti-IRAK, anti-TRAF6, anti-TAK1, and anti-TAB2 antibodies. (B) The interaction between TRAF6 and TAB2 is IRAK dependent. Extracts of 293 cells (wild type [WT]), IRAK-deficient cells (I1A), and I1A cells stably transfected with IRAK (I1A-IRAK), either untreated or treated with IL-1 for 10 min were immunoprecipitated with anti-TAB2 antibody and probed with anti-TRAF6 antibody. (C) Domains of IRAK required for the interaction of IRAK-TRAF6 with TAK1. Extracts of 293 cells (WT) and IRAK-deficient cells I1A, stably transfected with different IRAK deletion mutants (dDD, dUD, dKD, dC2, dC1C2, and DD+UD+C1), either untreated or treated with IL-1 for 10 min, were immunoprecipitated with anti-TAK1 antibody and probed with anti-IRAK, anti-TRAF6, and anti-TAK1 antibodies.
FIG. 5.
TAK1, TAB1, and TAB2 are preassociated on the membrane. (A) TAK1, TAB1, and TAB2 are preassociated. Extracts of 293 cells (wild type [WT]) and IRAK-deficient cells (I1A), either untreated or treated with IL-1, were immunoprecipitated with anti-TAK1 and anti-TAB2 antibodies and subjected to Western blot analyses with anti-TAB2, anti-TAK1, and anti-TAB1 antibodies. Preimmune serum (Pre) was used as a control. (B) TAK1, TAB1, and TAB2 form a complex on the membrane. Whole-cell extract (WCE) (prepared with SDS lysis buffer) of 293 cells and membrane and cytosolic fractions prepared from 293 cells with or without Triton (S-100, soluble fraction; S-100/0.5% Triton, soluble fraction prepared with 0.05% Triton; P-100, particulate fraction) were analyzed by the Western blot method with anti-TAB2, anti-TAB1, and anti-TAK1 antibodies. The membrane and cytosolic fractions were prepared in the same volume. Twice as much membrane fraction was loaded compared to the cytosolic fraction. The P-100 fractions were also resuspended in Triton lysis buffer, immunoprecipitated with anti-TAB2 antibody (P-100/IP: TAB2 ab), and analyzed with the same antibodies.
FIG. 6.
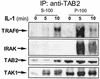
Formation of complex II on the membrane and translocation of complex III to the cytosol. Membrane (P-100) and cytosolic (S-100) fractions, prepared from 293 cells either untreated or treated with IL-1 for 5 or 10 min, were immunoprecipitated with anti-TAB2 antibody and subjected to Western blot analyses with anti-TRAF6, anti-IRAK, anti-TAB2, and anti-TAK1 antibodies. The P-100 fractions were resuspended in Triton lysis buffer before immunoprecipitation (IP).
FIG. 7.
TAK1, TAB1, and TAB2 are phosphorylated on the membrane, and TAK1 is activated in the cytosol. Extracts of 293 cells, either untreated or treated with IL-1 for the indicated times, were immunoprecipitated (IP) with anti-TAK1 antibody. The immunoprecipitates were then incubated with 1 μg of bacterially expressed HA-MKK6 in a kinase buffer containing γ-32P-labeled ATP at 25°C for 2 min. Samples were separated by SDS-PAGE and transferred to membranes, and the proteins were visualized by autoradiography. The same membrane was probed with anti-TAK1 antibody. IB, immunoblotting.
FIG. 8.
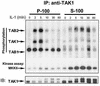
In vitro activation of TAK1. S-100 and Triton-solubilized P-100 fractions from 293 cells, treated with IL-1 for 2 min, were immunoprecipitated with anti-TAK1 antibody. The immunoprecipitates were incubated (+) or not incubated (−) with 50 μl of S-100 or Triton-solubilized P-100 from untreated 293 cells at 30°C for 10 min, washed with Triton lysis buffer, and then subjected to a TAK1 kinase assay using HA-MKK6 as a substrate. The samples were also studied by use of a Western blot probed with anti-TAK antibody.
References
- Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. - PubMed
- Barnes, P. J., and M. Karin. 1997. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336:1066-1071. - PubMed
- Burns, K., J. Clatworthy, L. Martin, F. Martinon, C. Plumpton, B. Maschera, A. Lewis, K. Ray, J. Tschopp, and F. Volpe. 2000. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat. Cell Biol. 2:346-351. - PubMed
- Cao, Z., W. J. Henzel, and X. Gao. 1996. IRAK: a kinase associated with the interleukin-1 receptor. Science 271:1128-1131. - PubMed
- Cao, Z., J. Xiong, M. Takeuchi, T. Kurama, and D. V. Goeddel. 1996. TRAF6 is a signal transducer for interleukin-1. Nature 383:443-446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous