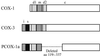COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression - PubMed (original) (raw)
COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression
N V Chandrasekharan et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Two cyclooxygenase isozymes, COX-1 and -2, are known to catalyze the rate-limiting step of prostaglandin synthesis and are the targets of nonsteroidal antiinflammatory drugs. Here we describe a third distinct COX isozyme, COX-3, as well as two smaller COX-1-derived proteins (partial COX-1 or PCOX-1 proteins). COX-3 and one of the PCOX-1 proteins (PCOX-1a) are made from the COX-1 gene but retain intron 1 in their mRNAs. PCOX-1 proteins additionally contain an in-frame deletion of exons 5-8 of the COX-1 mRNA. COX-3 and PCOX mRNAs are expressed in canine cerebral cortex and in lesser amounts in other tissues analyzed. In human, COX-3 mRNA is expressed as an approximately 5.2-kb transcript and is most abundant in cerebral cortex and heart. Intron 1 is conserved in length and in sequence in mammalian COX-1 genes. This intron contains an ORF that introduces an insertion of 30-34 aa, depending on the mammalian species, into the hydrophobic signal peptide that directs COX-1 into the lumen of the endoplasmic reticulum and nuclear envelope. COX-3 and PCOX-1a are expressed efficiently in insect cells as membrane-bound proteins. The signal peptide is not cleaved from either protein and both proteins are glycosylated. COX-3, but not PCOX-1a, possesses glycosylation-dependent cyclooxygenase activity. Comparison of canine COX-3 activity with murine COX-1 and -2 demonstrates that this enzyme is selectively inhibited by analgesic/antipyretic drugs such as acetaminophen, phenacetin, antipyrine, and dipyrone, and is potently inhibited by some nonsteroidal antiinflammatory drugs. Thus, inhibition of COX-3 could represent a primary central mechanism by which these drugs decrease pain and possibly fever.
Figures
Figure 1
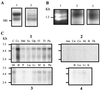
Northern blot analysis and RT-PCR. (A) Northern blot of canine cerebral cortex poly(A) RNA (1, 5.0 μg; 2, 2.5 μg) probed with (1) 32P-labeled canine COX-1 cDNA fragment and (2) 32P-labeled canine antisense oligonucleotide to intron 1. (B) PCR amplification of PCOX-1 in canine cerebral cortex. Lane 1, ethidium bromide-stained gel of amplified products corresponding to PCOX-1a containing intron 1 (upper band) and PCOX-1b (lower band) lacking intron 1; lane 2, Southern blot of the amplified products probed with antisense oligonucleotide to intron 1; lane 3, Southern blot using COX-3 cDNA as probe. (C) Human Multiple Tissue Northern blots (MTN) probed with a 32P-labeled human antisense oligonucleotide to intron 1 (HCI). The ≈5.2-kb mRNA was detected in blots 1–3 (adult tissues), and 4 (fetal tissues). Am, amygdala; B, brain; C, cerebellum: Cc, cerebral cortex; Fl, frontal lobe; Hi, hippocampus; Ht, heart; K, kidney; Lu, lung; Li, liver; M, skeletal muscle; Md, medulla; Cn, caudate nucleus; Op, occipital pole; P, placenta; Pn, pancreas; Pu, putamen; Sc, spinal cord; Th, thalamus; Tl, temporal lobe; Co, corpus callosum.
Figure 2
Structure of COX-3 and PCOX-1a. A schematic representation of the domains of COX-3 and PCOX-1 in comparison to COX-1. s, signal peptide; d1, dimerization domain/EGF-like domain 1; d2, dimerization domain 2; m, membrane binding domain; c, catalytic domain; i, 90-bp sequence encoded by intron 1. PCOX-1b is identical to PCOX-1a except that PCOX-1b lacks intron 1. Amino acid numbering is according to residues in sheep seminal vesicle COX-1.
Figure 3
Expression in insect cells. Western blots showing the expression of COX-3, PCOX-1a, and COX-1 in insect cells treated with (+) and without (−) tunicamycin (Upper). Arrows indicate glycosylated forms of COX-1 that are not present in cells treated with tunicamycin. Polyclonal antibodies to human and mouse COX-1 intron 1 sequence were used to probe the COX-3 and PCOX-1a blots, whereas a monoclonal antibody to ovine COX-1 (Cayman Chemical) was used to probe the mouse COX-1 blot. COX activity in insect cells expressing COX-3, PCOX-1a, and COX-1 (Lower). Cells were treated with (+) and without (−) tunicamycin.
Figure 4
Western blot of human aorta lysate probed with COX-1 and -3 antibodies. (A) Western blot (lanes 3–8, 20 μg total aorta protein each lane) probed with primary, secondary, or blocked antibodies as indicated. A solid horizontal arrow indicates the 65-kDa protein, an open arrow indicates the 53-kDa proteins, and an upward diagonal solid arrow indicates the 50-kDa protein. A single asterisk denotes unglycosylated canine COX-3, and a double asterisk denotes unglycosylated canine PCOX-1a. (B) Densitometric analysis of 65-, 53-, and 50-kDa proteins. Percent relative densitometric units (% rdu) were calculated by comparison to the signal from unblocked primary antibodies. The 50-kDa protein is not detected (n/d) by unblocked or blocked COX-3 polyclonal antibody (pAb).
Figure 5
Drug inhibition studies. The effects of acetaminophen (A and B), phenacetin (C), and dipyrone (D) on COX-1 (♦), COX-2 (●), and COX-3 (▪) activity in insect cells. COX activity was measured by the formation of PGE2 after exposure to exogenous 5 μM (B) or 30 μM (A, C, and D) arachidonic acid for 10 min. Data are expressed as mean ± SEM (n = 6–9).
Comment in
- Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum?
Warner TD, Mitchell JA. Warner TD, et al. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13371-3. doi: 10.1073/pnas.222543099. Epub 2002 Oct 8. Proc Natl Acad Sci U S A. 2002. PMID: 12374850 Free PMC article. Review. No abstract available. - COX-3: in the wrong frame in mind.
Dinchuk JE, Liu RQ, Trzaskos JM. Dinchuk JE, et al. Immunol Lett. 2003 Mar 3;86(1):121. doi: 10.1016/s0165-2478(02)00268-7. Immunol Lett. 2003. PMID: 12600754 No abstract available.
Similar articles
- Variants of cyclooxygenase-1 and their roles in medicine.
Simmons DL. Simmons DL. Thromb Res. 2003 Jun 15;110(5-6):265-8. doi: 10.1016/s0049-3848(03)00380-3. Thromb Res. 2003. PMID: 14592545 Review. - Nonsteroidal anti-inflammatory drugs, acetaminophen, cyclooxygenase 2, and fever.
Simmons DL, Wagner D, Westover K. Simmons DL, et al. Clin Infect Dis. 2000 Oct;31 Suppl 5:S211-8. doi: 10.1086/317517. Clin Infect Dis. 2000. PMID: 11113025 Review. - Determination of expression of cyclooxygenase-1 and -2 isozymes in canine tissues and their differential sensitivity to nonsteroidal anti-inflammatory drugs.
Wilson JE, Chandrasekharan NV, Westover KD, Eager KB, Simmons DL. Wilson JE, et al. Am J Vet Res. 2004 Jun;65(6):810-8. doi: 10.2460/ajvr.2004.65.810. Am J Vet Res. 2004. PMID: 15198222 - Acetaminophen-induced hypothermia in mice is mediated by a prostaglandin endoperoxide synthase 1 gene-derived protein.
Ayoub SS, Botting RM, Goorha S, Colville-Nash PR, Willoughby DA, Ballou LR. Ayoub SS, et al. Proc Natl Acad Sci U S A. 2004 Jul 27;101(30):11165-9. doi: 10.1073/pnas.0404185101. Epub 2004 Jul 19. Proc Natl Acad Sci U S A. 2004. PMID: 15263079 Free PMC article. - Human cyclooxygenase-1b is not the elusive target of acetaminophen.
Censarek P, Freidel K, Hohlfeld T, Schrör K, Weber AA. Censarek P, et al. Eur J Pharmacol. 2006 Dec 3;551(1-3):50-3. doi: 10.1016/j.ejphar.2006.08.079. Epub 2006 Sep 12. Eur J Pharmacol. 2006. PMID: 17045584
Cited by
- The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings.
Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. Graham GG, et al. Inflammopharmacology. 2013 Jun;21(3):201-32. doi: 10.1007/s10787-013-0172-x. Epub 2013 May 30. Inflammopharmacology. 2013. PMID: 23719833 Review. - The role of aspirin in cancer prevention.
Thun MJ, Jacobs EJ, Patrono C. Thun MJ, et al. Nat Rev Clin Oncol. 2012 Apr 3;9(5):259-67. doi: 10.1038/nrclinonc.2011.199. Nat Rev Clin Oncol. 2012. PMID: 22473097 Review. - Impairment of aspirin antiplatelet effects by non-opioid analgesic medication.
Polzin A, Hohlfeld T, Kelm M, Zeus T. Polzin A, et al. World J Cardiol. 2015 Jul 26;7(7):383-91. doi: 10.4330/wjc.v7.i7.383. World J Cardiol. 2015. PMID: 26225198 Free PMC article. Review. - Single dose oral ibuprofen plus paracetamol (acetaminophen) for acute postoperative pain.
Derry CJ, Derry S, Moore RA. Derry CJ, et al. Cochrane Database Syst Rev. 2013 Jun 24;2013(6):CD010210. doi: 10.1002/14651858.CD010210.pub2. Cochrane Database Syst Rev. 2013. PMID: 23794268 Free PMC article. Review. - ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells.
Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, Dmitriev AA. Snezhkina AV, et al. Oxid Med Cell Longev. 2019 Aug 5;2019:6175804. doi: 10.1155/2019/6175804. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31467634 Free PMC article. Review.
References
- Botting R M. Clin Infect Dis. 2000;31:8202–8210. - PubMed
- Flower R J, Vane J R. Nature (London) 1972;240:410–411. - PubMed
- Sambrook J, Russell D. Molecular Cloning: A Laboratory Manual. 3rd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 2001.
- Hla T. Prostaglandins. 1996;51:81–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials