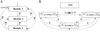Untangling the wires: a strategy to trace functional interactions in signaling and gene networks - PubMed (original) (raw)
Untangling the wires: a strategy to trace functional interactions in signaling and gene networks
Boris N Kholodenko et al. Proc Natl Acad Sci U S A. 2002.
Erratum in
- Proc Natl Acad Sci U S A 2002 Nov 12;99(23):15245
Abstract
Emerging technologies have enabled the acquisition of large genomics and proteomics data sets. However, current methodologies for analysis do not permit interpretation of the data in ways that unravel cellular networking. We propose a quantitative method for determining functional interactions in cellular signaling and gene networks. It can be used to explore cell systems at a mechanistic level or applied within a "modular" framework, which dramatically decreases the number of variables to be assayed. This method is based on a mathematical derivation that demonstrates how the topology and strength of network connections can be retrieved from experimentally measured network responses to successive perturbations of all modules. Importantly, our analysis can reveal functional interactions even when the components of the system are not all known. Under these circumstances, some connections retrieved by the analysis will not be direct but correspond to the interaction routes through unidentified elements. The method is tested and illustrated by using computer-generated responses of a modeled mitogen-activated protein kinase cascade and gene network.
Figures
Figure 1
A three-module cascade (A) and a gene network (B). The question marks stand for unknown connections and additional network components (e.g., uncharacterized genes), which can influence and in turn be affected by the known components.
Figure 2
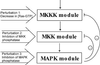
Kinetic scheme of the MAPK cascade. Feedback effects of MAPK on the rate of MKKK and MKK phosphorylation are shown schematically by dashed lines. MKKKK, MKKK kinase; P and PP, monophosphorylated and bisphosphorylated forms.
Figure 3
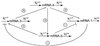
Kinetic scheme of a four-gene network. Arrows correspond to activation interactions, whereas lines with blunt ends represent inhibitions.
Figure 4
Global fractional responses obtained by simulating experimental perturbations to the MAPK cascade. Four global response matrices (Rp⋅100, designated by superscripts a–d) were generated by applying the following 12 parameter perturbations. a or b, 10 or 50% decrease, respectively, in: [Ras-GTP], perturbation to module 1; the catalytic activities of steps 5 and 6 (k and k
; see Table 1, which is published as supporting information on the PNAS web site), module 2; k
and k
, module 3. c or d, 10 or 50% decrease, respectively, in: [Ras-GTP], module 1; the maximal activities of steps 7 and 8 (V
and V
; see Table 1), module 2; V
and V
, module 3.
Figure 5
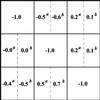
Retrieved “experimental” interaction maps (A) and known “theoretical” interaction map (B). Four experimental local interaction matrices (r) were retrieved from the data shown in Fig. 4. The response coefficients marked by superscripts a–d correspond to perturbations indicated in the legend to Fig. 4.
Figure 6
Revealed interactions between MAPK cascade modules. Responses of every module to perturbations to each single module were measured, and intermodular connections were discovered and quantified by using Eqs. 8 and 10.
Figure 7
Global fractional responses obtained by simulating experimental perturbations to the gene network shown in Fig. 3. Two global response matrices (Rp⋅100, designated by superscripts a and b) were generated by applying the following eight parameter perturbations. a or b, 30% decrease or 50% increase, respectively, in: the maximal activity of the transcription rate v (V
; see Table 2, which is published as supporting information on the PNAS web site), perturbation to module 1; V
, module 2; V
, module 3; and V
, module 4.
Figure 8
Retrieved experimental interaction maps (A) and known theoretical interaction map (B). Two experimental local interaction matrices (r) were retrieved from the data shown in Fig. 7. The response coefficients marked by superscript a and b correspond to perturbations indicated in the legend to Fig. 7.
Figure 9
Retrieved wiring for a three-gene network. Because gene 4 was an unknown component, (quasi)direct effects of gene 3 on genes 1 and 2 were revealed.
Figure 10
Interaction maps retrieved from incomplete data. Two experimental local interaction matrices (r) were retrieved from the global response matrix corresponding to the first three rows and columns of the matrices shown in Fig. 7. The response coefficients marked by superscripts a or b correspond to perturbations indicated in the legend to Fig. 7.
Similar articles
- Integrating Bayesian variable selection with Modular Response Analysis to infer biochemical network topology.
Santra T, Kolch W, Kholodenko BN. Santra T, et al. BMC Syst Biol. 2013 Jul 6;7:57. doi: 10.1186/1752-0509-7-57. BMC Syst Biol. 2013. PMID: 23829771 Free PMC article. - Modular response analysis of cellular regulatory networks.
Bruggeman FJ, Westerhoff HV, Hoek JB, Kholodenko BN. Bruggeman FJ, et al. J Theor Biol. 2002 Oct 21;218(4):507-20. J Theor Biol. 2002. PMID: 12384053 - Exhaustive analysis of the modular structure of the spliceosomal assembly network: a Petri net approach.
Bortfeldt RH, Schuster S, Koch I. Bortfeldt RH, et al. In Silico Biol. 2010;10(1):89-123. doi: 10.3233/ISB-2010-0419. In Silico Biol. 2010. PMID: 22430224 - Protein networking: insights into global functional organization of proteomes.
Pieroni E, de la Fuente van Bentem S, Mancosu G, Capobianco E, Hirt H, de la Fuente A. Pieroni E, et al. Proteomics. 2008 Feb;8(4):799-816. doi: 10.1002/pmic.200700767. Proteomics. 2008. PMID: 18297653 Review. - Docking interactions in protein kinase and phosphatase networks.
Reményi A, Good MC, Lim WA. Reményi A, et al. Curr Opin Struct Biol. 2006 Dec;16(6):676-85. doi: 10.1016/j.sbi.2006.10.008. Epub 2006 Oct 31. Curr Opin Struct Biol. 2006. PMID: 17079133 Review.
Cited by
- Reverse engineering a hierarchical regulatory network downstream of oncogenic KRAS.
Stelniec-Klotz I, Legewie S, Tchernitsa O, Witzel F, Klinger B, Sers C, Herzel H, Blüthgen N, Schäfer R. Stelniec-Klotz I, et al. Mol Syst Biol. 2012;8:601. doi: 10.1038/msb.2012.32. Mol Syst Biol. 2012. PMID: 22864383 Free PMC article. - Network quantification of EGFR signaling unveils potential for targeted combination therapy.
Klinger B, Sieber A, Fritsche-Guenther R, Witzel F, Berry L, Schumacher D, Yan Y, Durek P, Merchant M, Schäfer R, Sers C, Blüthgen N. Klinger B, et al. Mol Syst Biol. 2013;9:673. doi: 10.1038/msb.2013.29. Mol Syst Biol. 2013. PMID: 23752269 Free PMC article. - Encoding and decoding cellular information through signaling dynamics.
Purvis JE, Lahav G. Purvis JE, et al. Cell. 2013 Feb 28;152(5):945-56. doi: 10.1016/j.cell.2013.02.005. Cell. 2013. PMID: 23452846 Free PMC article. Review. - Extended kalman filter for estimation of parameters in nonlinear state-space models of biochemical networks.
Sun X, Jin L, Xiong M. Sun X, et al. PLoS One. 2008;3(11):e3758. doi: 10.1371/journal.pone.0003758. Epub 2008 Nov 19. PLoS One. 2008. PMID: 19018286 Free PMC article. - Sparsity as cellular objective to infer directed metabolic networks from steady-state metabolome data: a theoretical analysis.
Öksüz M, Sadıkoğlu H, Çakır T. Öksüz M, et al. PLoS One. 2013 Dec 31;8(12):e84505. doi: 10.1371/journal.pone.0084505. eCollection 2013. PLoS One. 2013. PMID: 24391961 Free PMC article.
References
- Smolen P, Baxter D A, Byrne J H. Am J Physiol. 1998;274:C531–C542. - PubMed
- Kholodenko B N, Demin O V, Moehren G, Hoek J B. J Biol Chem. 1999;274:30169–30181. - PubMed
- von Dassow G, Meir E, Munro E M, Odell G M. Nature (London) 2000;406:188–192. - PubMed
- Haugh J M, Wells A, Lauffenburger D A. Biotechnol Bioeng. 2000;70:225–238. - PubMed
- Hasty J, McMillen D, Isaacs F, Collins J J. Nat Rev Genet. 2001;2:268–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources