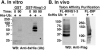A death-associated protein kinase (DAPK)-interacting protein, DIP-1, is an E3 ubiquitin ligase that promotes tumor necrosis factor-induced apoptosis and regulates the cellular levels of DAPK - PubMed (original) (raw)
A death-associated protein kinase (DAPK)-interacting protein, DIP-1, is an E3 ubiquitin ligase that promotes tumor necrosis factor-induced apoptosis and regulates the cellular levels of DAPK
Yijun Jin et al. J Biol Chem. 2002.
Abstract
Death-associated protein kinase (DAPK) is a multi-domain Ser/Thr protein kinase with an important role in apoptosis regulation. In these studies we have identified a DAPK-interacting protein called DIP-1, which is a novel multi-RING finger protein. The RING finger motifs of DIP-1 have E3 ligase activity that can auto-ubiquitinate DIP-1 in vitro. In vivo, DIP-1 is detected as a polyubiquitinated protein, suggesting that the intracellular levels of DIP-1 are regulated by the ubiquitin-proteasome system. Transient expression of DIP-1 in HeLa cells antagonizes the anti-apoptotic function of DAPK to promote a caspase-dependent apoptosis. These studies also demonstrate that DAPK is an in vitro and in vivo target for ubiquitination by DIP-1, thereby providing a mechanism by which DAPK activities can be regulated through proteasomal degradation.
Figures
Fig. 1. Cloning of a DIP-1
A, DAP kinase was immunoprecipitated from 35S-labeled COS cells under non-denaturing conditions. The co-precipitating proteins were visualized following SDS-PAGE by autoradiography. Numbers to the left indicate molecular weight standards. B, the deduced amino acid sequence of mouse DIP-1. The underscored residues represent the three RING fingers. C, schematic representation of the motifs predicted from the deduced sequence of DIP-1. Numbers indicate the beginning and end of each domain. ZnF, zinc finger; ANK, ankyrin repeats; RING, RING finger; COIL, _α_-helical coiled coil. D, multiple sequence alignment of RING finger motifs present in vertebrate and invertebrate inhibitor of apoptosis (IAP) proteins.
Fig. 2. Western (A) and Northern (B) blotting to detect DIP-1 mRNA and protein in mouse embryonic and adult tissues and cell lines
For Western blotting a polyclonal antibody prepared against recombinant DIP-1 was used to detect the endogenous 110-kDa protein corresponding to DIP-1. The last lane on the Western blot corresponds to recombinant 3× FLAG-tagged DIP-1 (Fl-DIP) expressed in transfected COS cells and shows that recombinant FL-DIP-1 co-migrates with ~110-kDa immunoreactive protein in cells and tissues. For the Northern blots, 20-_μ_g samples of total RNA isolated from the indicated mouse tissues were separated on a 1.2% agarose gel. An mRNA of ~4 kilobases (kB) is detected when the blots were probed with a 32P-labeled cRNA probe corresponding to bp 3370–3920 of the mouse DIP-1. Emb stem, embryonic stem; V. Deferens, vas deferens; MDCK, Madin-Darby canine kidney cells.
Fig. 3. Western blotting (WB) to detect co-immunoprecipitation (IP) of DIP-1 and DAPK
DAPK (upper panel) and DIP-1 (lower panel) were immunoprecipitated using either anti-DAPK or anti-DIP-1 antibody from HeLa cell extracts. The immunoprecipitates were fractionated by SDS-PAGE, and Western blots were reacted with the indicated anti-sera to detect DIP-1 or DAPK. The left lane (lysates) represents the immunoreactive protein present in 50 _μ_g of total cell extract. Immunoprecipitations using non-immune IgG were used in parallel as a control.
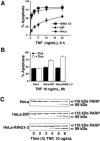
Fig. 4. Expression of DIP-1 promotes TNF-induced apoptosis
A, dose-response curves representing the levels of apoptosis in response to TNF treatment for 8 h. HeLa Tet-ON cell lines expressing either vector only (HeLa), full-length DIP-1 (HeLa-DIP-1), or the three RING fingers of DIP-1 (residues 815–998; RING 1–3) were treated for 24 h with doxycycline (DOX) to induce stable expression of DIP-1 or RING1–3 before TNF treatment. Percent apoptosis was quantified by determining the number of viable, non-apoptotic TNF-treated or control cells at the TNF concentrations indicated. B, levels of apoptosis in HeLa cells expressing DIP-1 or RING1–3 are increased in response to 10 ng/ml TNF for 8 h. Apoptosis levels were determined in cells expressing (+DOX) or not (–DOX) DIP-1 or RING1–3 or in parental HeLa cells. C, the temporal appearance of the 89-kDa poly-ADP-ribose polymerase (PARP) cleavage fragment is accelerated in HeLa cells expressing DIP-1 or RING1–3 compared with parental HeLa cells.
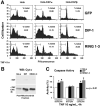
Fig. 5. Expression of DIP-1 or DIP-1-RING antagonizes the survival function of DAPK-β
A, HeLa cell lines overexpressing either DAPK-α or the more potent anti-apoptotic form of DAPK, DAPK-β, were transiently transfected with GFP-DIP-1 or GFP-RING1–3. At 24 h post-transfection, the cells were treated with TNF (10 ng/ml) and cyclohexamide (10 _μ_g/ml) for 4 h, then sorted to identify GFP-positive cells and analyzed for DNA content using propidium iodide (PI) staining. The gated region indicates sub-G1 levels of DNA (fragmented DNA). In the parental HeLa cells (left panels), expression of GFP-DIP-1 or GFP-RING1–3 enhances cell death compared with the GFP-only control. The right panels show that the death-promoting effect of DIP-1 or DIP-1-RING1–3 antagonizes the anti-apoptotic function of DAPK-β. B, HeLa cell lines expressing DIP-1 or RING1–3 have enhanced TNF-induced release of cytochrome c (Cyt c) from the mitochondria to the cytosol. WB, Western blot; CHX, cyclohexamide. C, HeLa cell lines expressing DIP-1 or RING1–3 have increased levels of caspase-3 and caspase-9 but not caspase-8. pNA, _p_-nitroanilide.
FIG. 6. DIP-1 is an E3 ligase in vitro and is ubiquitinated in vivo
A, Western blot (WB) to detect ubiquitinated RING1–3 after an in vitro ubiquitination assay. Recombinant RING1–3 was added to purified ubiquitin-activating enzyme (E1A) and ubiquitin-conjugating enzyme (Ubc5a) and incubated in the presence of ATP and His-ubiquitin (Ub). Anti-His antibody was used to detect polyubiquitinated RING1–3. B, Western blotting to detect FLAG-tagged RING1–3 (FL-RING1–3) or DIP-1 (FL-DIP-1) after treatment of cells with the proteasome inhibitor MG132. His-tagged ubiquitinated proteins were affinity-purified on Talon metal ion affinity beads, and anti-FLAG antibody was used to detect polyubiquitinated RING1–3 or DIP-1.
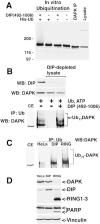
Fig. 7. DAPK is a substrate for ubiquitination by DIP-1
A, in vitro ubiquitination of DAPK by DIP-1. DAPK was immunoprecipitated (IP) from HeLa cells and incubated with recombinant DIP-1 (0.83 μ
m
)in the presence of E1, E2, ATP, and His-ubiquitin. B, HeLa cell lines expressing DIP-1 or RING1–3 were transfected with a vector for expression of ubiquitin. Ubiquitinated proteins were immunoprecipitated from the in vitro ubiquitination reactions using anti-ubiquitin antibody. After Western blotting (WB) anti-DAPK antibody was used to detect DAPK. Increased expression of DIP-1 or RING1–3 enhances the appearance of polyubiquitinated DAPK (Ub_n_-DAPK). CE, cell extract, C, HeLa cells were immunodepleted of endogenous DIP-1, and recombinant DIP-1-(492–1006) was added back to the cell lysate that also contained exogenous ATP, ubiquitin, and MG-132 (10 μ
m
). After 30 min of incubation, ubiquitinated proteins were immunoprecipitated and analyzed by Western blotting using anti-DAPK antibody. Significant levels of polyubiquitinated DAPK (Ub_n_-DAPK) appear only in reactions using native cell lysate (first lane) or lysate to which DIP-1 was added back (third lane). D, levels of endogenous DAPK were examined by Western blotting in extracts from the indicated HeLa cell lines after transient expression of ubiquitin. In HeLa cells expressing DIP-1, a modest decrease in the level of DAPK is observed, whereas in HeLa cells expressing RING1–3, a significant decrease in DAPK expression is detected. The Western blot in the upper panel was probed with anti-DAPK antibody and then successively with anti-DIP-1, anti-poly-ADP-ribose polymerase (PARP), and anti-vinculin antibody. Vinculin is used as a loading control to show equivalent amounts of cellular proteins in each lane.
Similar articles
- Regulation of death-associated protein kinase. Stabilization by HSP90 heterocomplexes.
Zhang L, Nephew KP, Gallagher PJ. Zhang L, et al. J Biol Chem. 2007 Apr 20;282(16):11795-804. doi: 10.1074/jbc.M610430200. Epub 2007 Feb 26. J Biol Chem. 2007. PMID: 17324930 Free PMC article. - Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death.
Suzuki Y, Nakabayashi Y, Takahashi R. Suzuki Y, et al. Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8662-7. doi: 10.1073/pnas.161506698. Epub 2001 Jul 10. Proc Natl Acad Sci U S A. 2001. PMID: 11447297 Free PMC article. - Ubiquitination of a novel deubiquitinating enzyme requires direct binding to von Hippel-Lindau tumor suppressor protein.
Li Z, Na X, Wang D, Schoen SR, Messing EM, Wu G. Li Z, et al. J Biol Chem. 2002 Feb 15;277(7):4656-62. doi: 10.1074/jbc.M108269200. Epub 2001 Dec 5. J Biol Chem. 2002. PMID: 11739384 - Post-translational regulation of the cellular levels of DAPK.
Gallagher PJ, Blue EK. Gallagher PJ, et al. Apoptosis. 2014 Feb;19(2):306-15. doi: 10.1007/s10495-013-0936-1. Apoptosis. 2014. PMID: 24185832 Review. - The DAP-kinase family of proteins: study of a novel group of calcium-regulated death-promoting kinases.
Shohat G, Shani G, Eisenstein M, Kimchi A. Shohat G, et al. Biochim Biophys Acta. 2002 Nov 4;1600(1-2):45-50. doi: 10.1016/s1570-9639(02)00443-0. Biochim Biophys Acta. 2002. PMID: 12445458 Review.
Cited by
- Death-associated protein kinase 1 as a therapeutic target for Alzheimer's disease.
Zhang T, Kim BM, Lee TH. Zhang T, et al. Transl Neurodegener. 2024 Jan 9;13(1):4. doi: 10.1186/s40035-023-00395-5. Transl Neurodegener. 2024. PMID: 38195518 Free PMC article. Review. - INHAT subunit SET/TAF-Iβ regulates PRC1-independent H2AK119 mono-ubiquitination via E3 ligase MIB1 in colon cancer.
Park J, Kim JY, Park JW, Kang JY, Oh H, Hahm JY, Chae YC, Chakravarti D, Seo SB. Park J, et al. NAR Cancer. 2023 Sep 22;5(3):zcad050. doi: 10.1093/narcan/zcad050. eCollection 2023 Sep. NAR Cancer. 2023. PMID: 37746636 Free PMC article. - The Role of Death-Associated Protein Kinase-1 in Cell Homeostasis-Related Processes.
Makgoo L, Mosebi S, Mbita Z. Makgoo L, et al. Genes (Basel). 2023 Jun 16;14(6):1274. doi: 10.3390/genes14061274. Genes (Basel). 2023. PMID: 37372454 Free PMC article. Review. - SMN post-translational modifications in spinal muscular atrophy.
Riboldi GM, Faravelli I, Rinchetti P, Lotti F. Riboldi GM, et al. Front Cell Neurosci. 2023 Feb 17;17:1092488. doi: 10.3389/fncel.2023.1092488. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36874214 Free PMC article. Review. - Comprehensive profiling of 1015 patients' exomes reveals genomic-clinical associations in colorectal cancer.
Zhao Q, Wang F, Chen YX, Chen S, Yao YC, Zeng ZL, Jiang TJ, Wang YN, Wu CY, Jing Y, Huang YS, Zhang J, Wang ZX, He MM, Pu HY, Mai ZJ, Wu QN, Long R, Zhang X, Huang T, Xu M, Qiu MZ, Luo HY, Li YH, Zhang DS, Jia WH, Chen G, Ding PR, Li LR, Lu ZH, Pan ZZ, Xu RH. Zhao Q, et al. Nat Commun. 2022 Apr 29;13(1):2342. doi: 10.1038/s41467-022-30062-8. Nat Commun. 2022. PMID: 35487942 Free PMC article.
References
- Glickman MH, Ciechanover A. Physiol. Rev. 2002;82:373–428. - PubMed
- Ciechanover A, Schwartz AL. Hepatology. 2002;35:3–6. - PubMed
- Ciechanover A, Orian A, Schwartz AL. Bioessays. 2000;22:442–451. - PubMed
- Ciechanover A, Orian A, Schwartz AL. J. Cell. Biochem. Suppl. 2000;34:40–51. - PubMed
- Schwartz AL, Ciechanover A. Annu. Rev. Med. 1999;50:57–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials