Constitutive nuclear factor-kappa B activity is required for central neuron survival - PubMed (original) (raw)
Constitutive nuclear factor-kappa B activity is required for central neuron survival
Asha L Bhakar et al. J Neurosci. 2002.
Abstract
The function of nuclear factor (NF)-kappaB within the developing and mature CNS is controversial. We have generated transgenic mice to reveal NF-kappaB transcriptional activity in vivo. As expected, constitutive NF-kappaB activity was observed within immune organs, and tumor necrosis factor-inducible NF-kappaB activity was present in mesenchymal cells. Intriguingly, NF-kappaB activity was also prominent in the CNS throughout development, especially within neocortex, olfactory bulbs, amygdala, and hippocampus. NF-kappaB in the CNS was restricted to neurons and blocked by overexpression of dominant-negative NF-kappaB-inducible kinase or the IkappaBalphaM super repressor. Blocking endogenous neuronal NF-kappaB activity in cortical neurons using recombinant adenovirus induced neuronal death, whereas induction of NF-kappaB activity increased levels of anti-apoptotic proteins and was strongly neuroprotective. Together, these data demonstrate a physiological role for NF-kappaB in maintaining survival of central neurons.
Figures
Fig. 1.
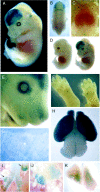
Transgene design and in vitro_validation of the κB-dependent β-galactosidase construct.A, The NF-κB reporter minigene contains three tandem HIV-LTR repeats upstream of the SV40 minimal promoter, an E. coli β-galactosidase cDNA modified to contain a mammalian Kozak consensus, an SV40 T-antigen-derived nuclear localization signal, and a polyA tract derived from the protamine I gene. B, HEK293 cells were transiently transfected with the minigene and induced with DMEM + TNFα (5 ng/ml) or with DMEM alone (indicated as control) for 16 hr and analyzed for β-galactosidase activity.C, Primary MEFs derived from transgenic mice were incubated with (panels 2,4) or without (panels 1,3) TNFα (5 ng/ml) for 16 hr and then assessed for β-galactosidase activity. Cultures were counterstained with Hoechst 33342 (panels 3, 4) to show cell nuclei. D, MEFs were infected with 0, 5, 50, or 250 MOI of recombinant IκBαM adenovirus for 24 hr, and total cell lysates were prepared and analyzed by immunoblotting for IκBα. Wells that were mock infected or infected with 50 MOI of recombinant IκBαM adenovirus were exposed to TNFα (20 ng/ml) for 10 min. Endogenous IκBα is completely degraded by this treatment, but IκBαM is unaffected. E, Transgenic MEFs were incubated with 0, 0.5, 2.5, 5, and 25 ng/ml TNFα for 16 hr in the absence (white bars) or presence (black bars) of IκBαM adenovirus (∼50% infection efficiency). β-galactosidase activity was quantified using a chemiluminescent assay (Galacto-Star; Tropix). Each data point represents the average of six wells of a 24-well plate, and error bars indicate SD. Results were analyzed for statistical significance by ANOVA [Tukey honestly significant difference (HSD) multiple comparison], and statistically significant differences of_p < 0.001 are indicated by an_asterisk_.
Fig. 2.
β-galactosidase expression pattern in discrete locations in embryonic and adult transgenic reporter mice.A, Whole-mount X-gal staining of an E13 transgenic mouse shows high basal NF-κB activity in the telencephalon and along the roof plate of the midbrain. Facial staining is visible within the primordia of the vibrissae (5 parallel rows) and in the prominent tactile hair follicles. B, Dorsal view of E13 transgenic embryos shows staining at the roof plate of the midbrain and at the midbrain–hindbrain junction. C, Close up of thoracic region. NF-κB activity is present in mammary gland primordia and in the gonadal area. D, β-galactosidase staining in transgenic (right) and in control littermate (left). E, E16 transgenic embryo showing prominent staining in vibrissae of the snout, in the olfactory lobes, and in the developing eyelid. F, NF-κB activity in the pads of the plantar surface of the E16 hindpaw (left) and of the palmer surface of the E16 forepaw (right).G, NF-κB activity within nuclei, likely multinucleated muscle fibers, beneath superficial layers of P1 skin. H, Robust NF-κB activity in P1 cortex, olfactory lobes, and roof plate of the midbrain. I–K, Lymphoid organs from postnatal day (P) 60 transgenic mice were analyzed for β-galactosidase activity as described in Materials and Methods. Constitutive NF-κB activity was detected along the trachea and bronchial tubes (I), in the thoracic lymph nodes (J and indicated by arrows in_I_), and in the thymus (K).
Fig. 3.
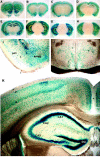
NF-κB activity in the adult brain.A–H, Serial sections (3 mm) of P180 transgenic brain were stained for β-galactosidase activity. Robust activity is visible in cortical layers 2, 4, and 5 (A–G), in the outer layers of the olfactory lobes (A), and in the islands of Calleja (olfactory tubercle) (A–C). Lower levels of β-galactosidase activity are present in the entorhinal and piriform cortices (D–F) and in the amygdala (D,E), claustrum (C–F), dentate gyrus, and hippocampus (D–G). I, NF-κB activity is present in the piriform–entorhinal cortex (piri) and is prominent in the amygdala (amyg). J, NF-κB activity is present in cells throughout the hypothalamus. K, NF-κB activity is prominent in the dentate gyrus (DG) and in CA1 and CA2 regions of the hippocampus. NF-κB activity is present in the CA3 region but lower than in CA1 and CA2 (also see D,E). Positive nuclei are also found in the stratum oriens, radiatum, and lacunosum moleculare of Ammon's horn. Within the cingulate and parietal cortex, positive nuclei are found in all cortical layers, but layers 2, 4, and 5 are stained most prominently.
Fig. 4.
NF-κB transcriptional activity is abundant in cultured primary cortical neurons. A, E16 primary cortical neurons derived from a heterozygote litter were grown for 10 DIV and then fixed and assessed for β-galactosidase activity.Arrows, Transgenic nuclei. B, E16 primary cortical neurons were immunostained for β-galactosidase and β-III-tubulin. β-galactosidase immunoreactivity is shown in_red_, β-III-tubulin is green, and nuclei stained with Hoescht 33342 are blue. C, Transgenic E16 cortical neurons derived from a heterozygote litter were infected with indicated MOIs of recombinant adenovirus expressing GFP, GFP and p65/RelA, or IκBαM for 48 hr, lysed, normalized for protein content, and analyzed for β-galactosidase content by immunoblot. Levels of β-III-tubulin assessed in parallel blots confirmed equivalent protein loading between lanes. D, Primary cortical neurons were infected with adenovirus encoding β-galactosidase (white bars) or IκBαM (black bars), and survival was measured by MTT conversion 48 hr later. Error bars indicate SD. Results were analyzed for statistical significance by ANOVA (Tukey HSD multiple comparison). Statistically significant differences of p < 0.001 are indicated by an asterisk. A–D, Each experiment was performed at least three times.
Fig. 5.
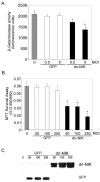
NIK signaling is required for NF-κB transcriptional activity and for neuronal viability in primary cortical neurons. A, E16 primary cortical neurons derived from a heterozygote litter were mock infected (gray bar) or infected with control GFP adenovirus (white bars) or dnNIK adenovirus (black bars) at 0.5 or 5 MOI and harvested 4 d later. Lysates were analyzed for β-galactosidase activity using a chemoluminescence assay (Tropix). β-galactosidase activity was significantly reduced in cells infected with 5 MOI of dnNIK (*p < 0.03). B,C, Cortical neurons were infected with 0 (gray bar), 50, 100, or 250 MOI of recombinant adenovirus encoding GFP (white bars) or dnNIK (black bars) for 72 hr and then analyzed for viability by MTT dye conversion (B) and for GFP and dnNIK expression by immunoblotting (C). β-galactosidase overexpression had no significant effect on neuronal survival, but overexpression of dnNIK reduced survival at each MOI tested (*p < 0.001). A,B, Six wells were analyzed per condition, and results were analyzed for statistical significance by ANOVA (Tukey HSD multiple comparison). A–C, Each experiment was repeated at least three times.
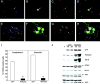
Fig. 6.
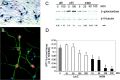
p65/RelA protects cortical neurons from apoptotic death. E15–16 cortical neurons were infected with 75 MOI of recombinant adenovirus encoding GFP alone (A–D) or with recombinant virus encoding both p65/RelA and GFP (E–H) for 24 hr. Cells were then exposed to etoposide (20 μ
m
) for an additional 18 hr and then fixed and analyzed for GFP fluorescence (B, F,green), for apoptosis using Hoescht 33342 nuclear staining (A, E, blue), and TUNEL labeling (C, G,red). D, H, Merged images of A–C and E–G, respectively. Cells infected with GFP alone (and uninfected cells) rapidly underwent apoptosis when exposed to etoposide, whereas neurons infected with p65/RelA were robustly viable under these conditions. I, Cells were infected with 75 MOI of adenovirus expressing either GFP or expressing GFP together with p65/RelA for 48 hr and then treated with camptothecin (20 μ
m
) or etoposide (20 μ
m
) for 18 hr. GFP-positive cells were scored for TUNEL-positive nuclei. Expression of p65/RelA conferred robust protection from apoptosis because of campthothecin (*p < 0.001) or etoposide (*p < 0.0001). At least 300 cells were assessed for each condition, and results were analyzed for statistical significance by Student's t test. J, E16 cortical neurons were either left uninfected or were infected with 75 MOI of recombinant adenovirus expressing GFP or expressing both GFP and p65/RelA for 48 hr. Neurons were then lysed and analyzed by immunoblot. Levels of endogenous IκBα, NFkB1, IAP1, IAP2, and Bcl-XL were specifically increased by p65/RelA overexpression.
Similar articles
- NFκB-inducing kinase inhibits NFκB activity specifically in neurons of the CNS.
Mao X, Phanavanh B, Hamdan H, Moerman-Herzog AM, Barger SW. Mao X, et al. J Neurochem. 2016 Apr;137(2):154-63. doi: 10.1111/jnc.13526. Epub 2016 Mar 15. J Neurochem. 2016. PMID: 26778773 Free PMC article. - Adenovirus-mediated expression of a dominant negative mutant of p65/RelA inhibits proinflammatory gene expression in endothelial cells without sensitizing to apoptosis.
Soares MP, Muniappan A, Kaczmarek E, Koziak K, Wrighton CJ, Steinhäuslin F, Ferran C, Winkler H, Bach FH, Anrather J. Soares MP, et al. J Immunol. 1998 Nov 1;161(9):4572-82. J Immunol. 1998. PMID: 9794384 - Inhibition of nuclear factor-kappaB activation induces apoptosis in cerebellar granule cells.
Piccioli P, Porcile C, Stanzione S, Bisaglia M, Bajetto A, Bonavia R, Florio T, Schettini G. Piccioli P, et al. J Neurosci Res. 2001 Dec 15;66(6):1064-73. doi: 10.1002/jnr.1251. J Neurosci Res. 2001. PMID: 11746438 - NF-kappaB signaling in neurite growth and neuronal survival.
Teng FY, Tang BL. Teng FY, et al. Rev Neurosci. 2010;21(4):299-313. doi: 10.1515/revneuro.2010.21.4.299. Rev Neurosci. 2010. PMID: 21086762 Review. - Cellular Specificity of NF-κB Function in the Nervous System.
Dresselhaus EC, Meffert MK. Dresselhaus EC, et al. Front Immunol. 2019 May 9;10:1043. doi: 10.3389/fimmu.2019.01043. eCollection 2019. Front Immunol. 2019. PMID: 31143184 Free PMC article. Review.
Cited by
- Argon inhalation attenuates retinal apoptosis after ischemia/reperfusion injury in a time- and dose-dependent manner in rats.
Ulbrich F, Schallner N, Coburn M, Loop T, Lagrèze WA, Biermann J, Goebel U. Ulbrich F, et al. PLoS One. 2014 Dec 23;9(12):e115984. doi: 10.1371/journal.pone.0115984. eCollection 2014. PLoS One. 2014. PMID: 25535961 Free PMC article. - Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2.
Bell KF, Al-Mubarak B, Martel MA, McKay S, Wheelan N, Hasel P, Márkus NM, Baxter P, Deighton RF, Serio A, Bilican B, Chowdhry S, Meakin PJ, Ashford ML, Wyllie DJ, Scannevin RH, Chandran S, Hayes JD, Hardingham GE. Bell KF, et al. Nat Commun. 2015 May 13;6:7066. doi: 10.1038/ncomms8066. Nat Commun. 2015. PMID: 25967870 Free PMC article. - Roles of HIF-1α, VEGF, and NF-κB in Ischemic Preconditioning-Mediated Neuroprotection of Hippocampal CA1 Pyramidal Neurons Against a Subsequent Transient Cerebral Ischemia.
Lee JC, Tae HJ, Kim IH, Cho JH, Lee TK, Park JH, Ahn JH, Choi SY, Bai HC, Shin BN, Cho GS, Kim DW, Kang IJ, Kwon YG, Kim YM, Won MH, Bae EJ. Lee JC, et al. Mol Neurobiol. 2017 Nov;54(9):6984-6998. doi: 10.1007/s12035-016-0219-2. Epub 2016 Oct 26. Mol Neurobiol. 2017. PMID: 27785755 - The effects of chronic, continuous β-funaltrexamine pre-treatment on lipopolysaccharide-induced inflammation and behavioral deficits in C57BL/6J mice.
Hodge K, Buck DJ, Das S, Davis RL. Hodge K, et al. J Inflamm (Lond). 2024 Sep 2;21(1):33. doi: 10.1186/s12950-024-00407-9. J Inflamm (Lond). 2024. PMID: 39223594 Free PMC article. - Identification of novel small molecule activators of nuclear factor-κB with neuroprotective action via high-throughput screening.
Manuvakhova MS, Johnson GG, White MC, Ananthan S, Sosa M, Maddox C, McKellip S, Rasmussen L, Wennerberg K, Hobrath JV, White EL, Maddry JA, Grimaldi M. Manuvakhova MS, et al. J Neurosci Res. 2011 Jan;89(1):58-72. doi: 10.1002/jnr.22526. J Neurosci Res. 2011. PMID: 21046675 Free PMC article.
References
- Bakalkin G, Yakovleva T, Terenius L. NF-kappa B-like factors in the murine brain. Developmentally-regulated and tissue-specific expression. Brain Res Mol Brain Res. 1993;20:137–146. - PubMed
- Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci USA. 1995;92:9328–9332. - PMC - PubMed
- Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. - PubMed
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. - PubMed
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials