The neuronal nitric oxide synthase gene is critically involved in neurobehavioral effects of alcohol - PubMed (original) (raw)
The neuronal nitric oxide synthase gene is critically involved in neurobehavioral effects of alcohol
Rainer Spanagel et al. J Neurosci. 2002.
Abstract
In the present study, we describe a new role of the neuronal nitric oxide synthase (nNOS) gene in the regulation of alcohol drinking behavior. Mice deficient in the nNOS gene (nNOS -/-) and wild-type control mice were submitted to a two-bottle free-choice procedure with either water or increasing concentrations of alcohol (2-16%) for 6 weeks. nNOS -/- mice did not differ in consumption and preference for low alcohol concentrations from wild-type animals; however, nNOS -/- mice consumed sixfold more alcohol from highly concentrated alcohol solutions than wild-type mice. Taste studies with either sucrose or quinine solutions revealed that alcohol intake in nNOS -/- and wild-type mice is associated, at least in part, with sweet solution intake but not with the taste of bitterness. When compared with wild-type mice, the nNOS -/- mice were found to be less sensitive to the sedative effects of ethanol as measured by shorter recovery time from ethanol-induced sleep and did not develop rapid tolerance to ethanol-induced hypothermia, although plasma ethanol concentrations were not significantly different from those of controls. Our findings contrast with previous reports that showed that nonselective NOS inhibitors decrease alcohol consumption. However, because alcohol consumption was suppressed in wild-type as well as nNOS -/- mice by the NOS inhibitor N(G)-nitro-L-arginine methyl ester, we conclude that the effect of nonselective NOS inhibitors on alcohol drinking is not mediated by nNOS. Other NOS isoforms, most likely in the periphery or other splice variants of the NOS gene, might contribute to the effect of nonselective NOS inhibitors on alcohol drinking. In summary, the nNOS gene is critically involved in the regulation of neurobehavioral effects of alcohol.
Figures
Fig. 1.
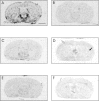
In situ hybridization analysis of nNOSα, β, and γ mRNA levels in the ventral striatum in wild-type and knock-out animals. Autoradiographs depicting the in situ hybridization of nNOS splice variant probes α (A, B), β (C,D), and γ (E, F) in horizontal brain sections in wild-type mice (A,C, E), and NOSα −/− mice (B, D, F). Scale bar, 10 mm. nNOSα mRNA signals were completely abolished in knock-out mice, whereas nNOSβ and nNOSγ mRNA were still detectable. Moreover, nNOSβ mRNA seems to be slightly increased in nNOSα −/− animals, especially within striatum (filled arrow) and cortex (open arrow). CPu, Caudate putamen; FrP, frontoparietal cortex.
Fig. 2.
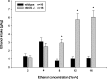
Acquisition of alcohol self-administration in nNOS knock-out and wild-type mice. Mice had the free choice between tap water and a 2% (v/v) alcohol solution for 3 d. For days 4–6, the ethanol concentration was increased to 4%, for days 7–16 it was increased to 8%, for days 16–25 it was increased to 12%, and for days 25–44 it was increased to 16%. Bars show mean + SEM ethanol intake per day in grams per kilogram body weight. *p < 0.05 indicates a significant difference between wild-type and nNOS −/− mice in ethanol consumption per day.
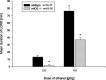
Fig. 3.
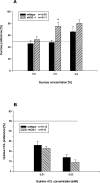
A, Sucrose preference in nNOS knock-out and wild-type mice. B, Quinine aversion in nNOS knock-out and wild-type mice. Sucrose (0.5, 2.5, and 5% w/v) and quinine (0.01 and 0.02 m
m
) solution intake was measured in a two-bottle free-choice test in alcohol-naïve mice.Bars show mean ± SE sucrose or quinine preference. *p < 0.05 indicates a significant difference between wild-type and nNOS knock-out mice.
Fig. 4.
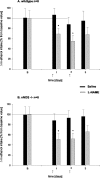
Alcohol intake in wild-type (A) and nNOS knock-out (B) mice after treatment with the NOS inhibitor
l
-NAME. Because basal alcohol intake differed between both genotypes, all values were normalized and are now given as percentage.
l
-NAME was injected at days 1 and 2 (indicated by ↑), and _bars_represent the difference in alcohol intake + SE compared with the basal intake (indicated by B). *p < 0.05 indicates a significant difference from basal intake.
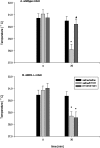
Fig. 5.
Sensitivity to the hypnotic effect of ethanol assessed by the measurement of loss of righting response (LORR) in wild-type and nNOS −/− mice. Ethanol (2.5 and 4.5 gm/kg, i.p.) was injected, and the time to regain the righting effect was measured. Each value is the mean + SE. *p < 0.05 indicates a significant difference from wild-type mice.
Fig. 6.
Development of rapid tolerance to ethanol-induced hypothermia in wild-type mice (A) but not in nNOS knock-out mice (B). The figure represents the effects of a second ethanol injection (3.5 gm/kg, i.p.) in animals that received 24 hr before the first ethanol injection (3.5 gm/kg, i.p.).Bars represent the mean + SE of the ventral surface temperature measured by an infrared thermometer in eight to nine mice per group. *p < 0.05 indicates significant differences from the basal values (time point 0).
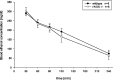
Fig. 7.
Blood ethanol elimination curve in nNOS knock-out and wild-type mice. nNOS−/− and wild-type mice received an intraperitoneal injection of 3.5 gm/kg ethanol, and blood samples were taken from the tail vein at different time points. Values are means ± SE in milligrams of ethanol per deciliters of blood.
Similar articles
- Contribution of nitric oxide synthase isoforms to cholinergic vasodilation in murine retinal arterioles.
Gericke A, Goloborodko E, Sniatecki JJ, Steege A, Wojnowski L, Pfeiffer N. Gericke A, et al. Exp Eye Res. 2013 Apr;109:60-6. doi: 10.1016/j.exer.2013.01.012. Epub 2013 Feb 19. Exp Eye Res. 2013. PMID: 23434456 - Effects of ethanol on neutrophil recruitment and lung host defense in nitric oxide synthase I and nitric oxide synthase II knockout mice.
Greenberg SS, Ouyang J, Zhao X, Parrish C, Nelson S, Giles TD. Greenberg SS, et al. Alcohol Clin Exp Res. 1999 Sep;23(9):1435-45. Alcohol Clin Exp Res. 1999. PMID: 10512307 - Role of nNOS in blood pressure regulation in eNOS null mutant mice.
Kurihara N, Alfie ME, Sigmon DH, Rhaleb NE, Shesely EG, Carretero OA. Kurihara N, et al. Hypertension. 1998 Nov;32(5):856-61. doi: 10.1161/01.hyp.32.5.856. Hypertension. 1998. PMID: 9822444 - Neuronal NOS: gene structure, mRNA diversity, and functional relevance.
Wang Y, Newton DC, Marsden PA. Wang Y, et al. Crit Rev Neurobiol. 1999;13(1):21-43. doi: 10.1615/critrevneurobiol.v13.i1.20. Crit Rev Neurobiol. 1999. PMID: 10223522 Review. - Ethanol metabolism and effects: nitric oxide and its interaction.
Deng XS, Deitrich RA. Deng XS, et al. Curr Clin Pharmacol. 2007 May;2(2):145-53. doi: 10.2174/157488407780598135. Curr Clin Pharmacol. 2007. PMID: 18690862 Free PMC article. Review.
Cited by
- Impact of Genetic Variation on Stress-Related Ethanol Consumption.
Mulligan MK, Lu L, Cavigelli SA, Mormède P, Terenina E, Zhao W, Williams RW, Jones BC. Mulligan MK, et al. Alcohol Clin Exp Res. 2019 Jul;43(7):1391-1402. doi: 10.1111/acer.14073. Epub 2019 May 21. Alcohol Clin Exp Res. 2019. PMID: 31034606 Free PMC article. - Deletion of Prkcz increases intermittent ethanol consumption in mice.
Lee AM, Zou ME, Lim JP, Stecher J, McMahon T, Messing RO. Lee AM, et al. Alcohol Clin Exp Res. 2014 Jan;38(1):170-8. doi: 10.1111/acer.12211. Epub 2013 Aug 1. Alcohol Clin Exp Res. 2014. PMID: 23905844 Free PMC article. - The Interplay of Inflammatory Processes and Cognition in Alcohol Use Disorders-A Systematic Review.
Coppens V, Morrens M, Destoop M, Dom G. Coppens V, et al. Front Psychiatry. 2019 Sep 12;10:632. doi: 10.3389/fpsyt.2019.00632. eCollection 2019. Front Psychiatry. 2019. PMID: 31572234 Free PMC article. - Inhibition of phosphodiesterase 2 by Bay 60-7550 decreases ethanol intake and preference in mice.
Shi J, Liu H, Pan J, Chen J, Zhang N, Liu K, Fei N, O'Donnell JM, Zhang HT, Xu Y. Shi J, et al. Psychopharmacology (Berl). 2018 Aug;235(8):2377-2385. doi: 10.1007/s00213-018-4934-4. Epub 2018 Jun 7. Psychopharmacology (Berl). 2018. PMID: 29876622 - Genes and Alcohol Consumption: Studies with Mutant Mice.
Mayfield J, Arends MA, Harris RA, Blednov YA. Mayfield J, et al. Int Rev Neurobiol. 2016;126:293-355. doi: 10.1016/bs.irn.2016.02.014. Int Rev Neurobiol. 2016. PMID: 27055617 Free PMC article. Review.
References
- Adams ML, Meyer ER, Sewing BN, Cicero TJ. Effects of nitric oxide-related agents on alcohol narcosis. Alcohol Clin Exp Res. 1994;18:969–975. - PubMed
- Azad SC, Marsicano G, Eberlein I, Putzke J, Zieglgänsberger W, Spanagel R, Lutz B. Differential role of the nitric oxide pathway on Δ9-THC-induced central nervous system effects in the mouse. Eur J Neurosci. 2001;13:561–568. - PubMed
- Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;6:175–195. - PubMed
- Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases