Molecular anatomy of antigen-specific CD8(+) T cell engagement and synapse formation in vivo - PubMed (original) (raw)
Molecular anatomy of antigen-specific CD8(+) T cell engagement and synapse formation in vivo
Dorian B McGavern et al. Nat Immunol. 2002 Oct.
Abstract
Antigen-specific CD8(+) T cells are required for the clearance of most viral infections and several cancers. However, it is not clear in vivo whether CD8(+) T cells can engage multiple targets simultaneously, engagement results in the formation of an immunologic synapse or molecules involved in CD8 function are redistributed to the synapse. We used here high-resolution microscopy to visualize interactions between virus-specific effectors and target cells in vivo. Using either in situ tetramer staining or green fluorescent protein-labeled virus-specific T cells, we have shown that a single CD8(+) T cell can engage two or three targets, a synapse occurs at the site of engagement and molecules involved in attachment (lymphocyte function-associated antigen 1), signaling (Lck) and lytic activity (perforin) are differentially positioned on the T cell. In addition, we have established an in vivo approach for assessing the intricacies of antigen-specific T cell activation, migration, engagement, memory and other defining elements of adaptive immunity.
Figures
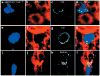
Figure 1. Tetramer staining of virus-specific CD8+ T cells in the spleens and CNS of GP33 TCR–Tg mice
Antigen-specific CD8+ T cells stained with an anti-CD8 and MHC class I tetramers were analyzed by (a) flow cytometry or (b–j) confocal microscopy. (a) By flow cytometry, 94% of CD8+T cells from the spleens of a naïve GP33 TCR–Tg mouse were tetramer+. In a nontransgenic B6 control, none of the CD8+ T cells were tetramer+. (b–d) Db-GP(33–41)-specific (green) CD8+ T cells (red) were detected in the splenic white pulp of naïve GP33 TCR–Tg mice. Overlapping fluorescence in the merged image appears in yellow. Of these T cells, 82% were tetramer+. (e–g) None of the CD8+ T cells from GP33 TCR–Tg mice were labeled with the control tetramer Db-NP(396–404). (h–j) Interactions between CD8+ (red) and Db-GP(33–41)–specific (green) effectors and LCMV-infected targets (blue) were visualized in the CNS of GP33 TCR–Tg mice on day 4 after LCMV infection. Red circles represent individual CD8+T cells; overlapping fluorescence between CD8 (red) and tetramer (green) appears in yellow. (h) Note the foci of tetramer staining (arrows) present around the cell membrane of a CTL in the area of LCMV infection. (h,i) TCR polarization to the CTL–target cell interface was also observed (arrowheads). (h, inset) More homogenous TCR distribution was observed on CTLs not engaged with virus-infected targets in the CNS. (j, arrows and inset) In rare instances (<1%), CTLs extended TCR-coated protrusions that partially wrapped around the virus-infected target.
Figure 2. Visualization of antiviral immunity in the CNS of B6 mice during the induction of lethal meningitis
(a) Mononuclear cells were isolated from the CNS and spleens of B6 mice on days 4, 5 and 6 after infection and the frequencies of Db-GP(33–41)-specific CD8+ T cells were calculated. Percentage numbers of cells are indicated (n = 4). (b–d) Brain sections were cut from symptomatic B6 mice on day 6 after infection and analyzed by in situ tetramer staining (green) and confocal microscopy. Many CD8+T cells (red) were present in the meninges, ependyma and choroid plexus. (b–d) Dense meningeal infiltrate in which four CD8+ T cells were Db-GP(33–41)+ (arrows). The frequency of tetramer+ cells was calculated by sampling CNS infiltrates from three infected mice. (e) With three-color analyses,TCR focusing (yellow) was observed at the interface between a single CTL (red) and an LCMV-infected target (blue).Yellow represents overlap between the tetramer (green) and CD8 (red) stains. Note the presence of tetramer staining at the interface (arrows), but not around the remaining CD8+ T cell membrane. (f) CD8+ T cells (red) interacted with up to three LCMV-infected (green) target cells (asterisks) in the CNS. Overlapping membrane between the CTL and infected targets appears in yellow.A tetramer stain is not shown. (g) Tetramer+ cells were also found interacting with multiple targets (asterisks). Note the foci of tetramer staining (yellow) around the cell membrane of a CTL (red) that is engaged with two targets (blue). The TCR is not polarized toward either target.
Figure 3. Expansion and migration of GFP+ Db-GP(33–41)-specific T cells after an i.c. infection
(a) On day 5 after infection, mononuclear cells were isolated from the spleens and CNS of B6 mice that were given GFP+ Db-GP(33–41)-specific T cells. Cells were stained with anti-CD8 and Db-GP(33–41) tetramers and analyzed by flow cytometry. Percentage numbers of cells are indicated (n = 4). (b–f) Reconstructions of coronal brain tissue sections were done on day 5 after infection to visualize the distribution of infiltrating GFP+ effector cells in relation to LCMV-infected targets. An overlay of nuclei (blue), LCMV (red) and GFP+ effectors (green) was generated with three-color immunofluorescence and image analysis software. The overlay (b) revealed that GFP+ effectors (d) localized primarily to the sites of LCMV infection (c). High-magnification images of b show the distribution of GFP+ effectors around the LCMV ependyma (e) and meninges (f). Each green dot represents a single antigen-specific CD8+ T cell.
Figure 4. Cellular reorganization of Db-GP(33–41)-specific T cells juxtaposed to LCMV-infected targets
The cell membrane distribution of (a–d) LFA-1, (e–h) Lck and (i–l) perforin on GFP+ Db-GP(33–41)-specific T cells (blue) engaged with LCMV-infected targets (red) was evaluated by confocal microscopy on day 5 after infection. (a–d) Distribution of LFA-1 (green) on a single Db-GP(33–41)-specific CTL. Note the polarization of LFA-1 staining toward the LCMV-infected target cell (arrows). (e–h) The distribution of Lck in a CTL surrounded almost entirely by LCMV-infected targets. The GFP+ cell showed dense Lck staining on one side of the cell membrane (arrows). (i–l) An example of polarized perforin staining at the interface between a CTL and virus-infected target. Nearly all the perforin staining (green) is localized on two LCMV-infected targets (arrows). Asterisks indicate the LCMV-infected targets. Overlapping LCMV (red) and perforin (green) signals appear in yellow.
Figure 5. Patterns of interfacial LFA-1, Lck and perforin staining on Db-GP(33–41)-specific T cells in the CNS
Representative staining patterns (all in green) are shown for (a,b) LFA-1, (c,d) Lck and (e) perforin. (f) The distribution of LFA-1 and Lck was quantified on conjugates and nonconjugates in the CNS.Asterisks denote a statistical difference between conjugates and nonconjugates (P < 0.001). (b) Polarization of LFA-1 (arrows) was observed on 64% (23/36) of the conjugates of Db-GP(33–41)-specific T cells (blue) and LCMV-infected targets (red). (a) In contrast, polarized LFA-1 was only observed on 7% (2/29) of the CTLs not engaged with LCMV-infected targets. (d) Aggregation of Lck was observed for 55% (21/38) of the conjugates (arrows). (c) This pattern was observed on 4% (2/45) of nonconjugates. Complete polarization of Lck was never observed. (e) CTL engagement of two LCMV-infected targets (asterisks). Note the delivery of perforin on both infected targets (arrows), which are in engaged at opposite ends of the CTL.The overlap between perforin (green) and LCMV (red) appears in yellow. Perforin staining can also be observed inside the CTL.
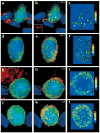
Figure 6. LFA-1 and Lck 3D localization on Db-GP(33–41)-specific T cells in the CNS
Maximal projections of 3D datasets show the total cellular distribution of (a–f) LFA-1 and (g–l) Lck, which was examined on both CNS (a–c, g–i) conjugates and (d–f, j–l) nonconjugates. Pseudocolored contour plots show the distribution of (c,f) LFA-1 and (i,l) Lck on the GFP+ Db-GP(33–41)-specific T cells.All maximal projections showed Db-GP(33–41)-specific T cells (green) and nuclei (blue). The left-hand panels show the virus in red; the middle panels show LFA-1 or Lck in red. (a) A Db-GP(33–41)-specific T cell in juxtaposition with at least three LCMV-infected targets (asterisks). (b,c) Polarization of LFA-1 was observed at the interface with each of these virus-infected targets (arrowheads) and on a process that extended from the CTL (arrow). (b) Inset shows the distribution of LFA-1 at the interface with one of the virus-infected targets. (d–f) A nonconjugate in the CNS; (e,f) note the presence of evenly distributed LFA-1 clusters around the cell membrane. (g) Another CTL–target cell conjugate. (h,i) Lck aggregation was observed at the interface between the CTL and the virus-infected target (arrowheads). (j–l) A more homogenous distribution of Lck was observed on nonconjugates.
Similar articles
- Homeostatic regulation of CD8+ T cells by perforin.
Kägi D, Odermatt B, Mak TW. Kägi D, et al. Eur J Immunol. 1999 Oct;29(10):3262-72. doi: 10.1002/(SICI)1521-4141(199910)29:10<3262::AID-IMMU3262>3.0.CO;2-A. Eur J Immunol. 1999. PMID: 10540338 - CD8(+)-T-cell response to secreted and nonsecreted antigens delivered by recombinant Listeria monocytogenes during secondary infection.
Tvinnereim AR, Hamilton SE, Harty JT. Tvinnereim AR, et al. Infect Immun. 2002 Jan;70(1):153-62. doi: 10.1128/IAI.70.1.153-162.2002. Infect Immun. 2002. PMID: 11748177 Free PMC article. - Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections.
Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Bachmann MF, et al. Eur J Immunol. 2007 Jun;37(6):1502-12. doi: 10.1002/eji.200637023. Eur J Immunol. 2007. PMID: 17492805 - Cytotoxic T cell effector and memory function in viral immunity.
Doherty PC. Doherty PC. Curr Top Microbiol Immunol. 1996;206:1-14. doi: 10.1007/978-3-642-85208-4_1. Curr Top Microbiol Immunol. 1996. PMID: 8608712 Review. No abstract available.
Cited by
- Visualization of Cell-Cell Interaction Contacts: Synapses and Kinapses.
Dustin ML. Dustin ML. Self Nonself. 2011 Apr;2(2):85-97. doi: 10.4161/self.2.2.17931. Epub 2011 Apr 1. Self Nonself. 2011. PMID: 22299060 Free PMC article. - A model for the interplay of receptor recycling and receptor-mediated contact in T cells.
Arkhipov SN, Maly IV. Arkhipov SN, et al. PLoS One. 2007 Jul 25;2(7):e633. doi: 10.1371/journal.pone.0000633. PLoS One. 2007. PMID: 17653260 Free PMC article. - Rapid formation of extended processes and engagement of Theiler's virus-infected neurons by CNS-infiltrating CD8 T cells.
McDole JR, Danzer SC, Pun RY, Chen Y, Johnson HL, Pirko I, Johnson AJ. McDole JR, et al. Am J Pathol. 2010 Oct;177(4):1823-33. doi: 10.2353/ajpath.2010.100231. Epub 2010 Sep 2. Am J Pathol. 2010. PMID: 20813972 Free PMC article. - High-resolution, noninvasive longitudinal live imaging of immune responses.
Abdulreda MH, Faleo G, Molano RD, Lopez-Cabezas M, Molina J, Tan Y, Echeverria OA, Zahr-Akrawi E, Rodriguez-Diaz R, Edlund PK, Leibiger I, Bayer AL, Perez V, Ricordi C, Caicedo A, Pileggi A, Berggren PO. Abdulreda MH, et al. Proc Natl Acad Sci U S A. 2011 Aug 2;108(31):12863-8. doi: 10.1073/pnas.1105002108. Epub 2011 Jul 18. Proc Natl Acad Sci U S A. 2011. PMID: 21768391 Free PMC article. - The synapse and cytolytic machinery of cytotoxic T cells.
Jenkins MR, Griffiths GM. Jenkins MR, et al. Curr Opin Immunol. 2010 Jun;22(3):308-13. doi: 10.1016/j.coi.2010.02.008. Epub 2010 Mar 11. Curr Opin Immunol. 2010. PMID: 20226643 Free PMC article. Review.
References
- Zinkernagel RM, Doherty PC. Restriction of in vitro T cell–mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–702. - PubMed
- Kagi D, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. - PubMed
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. - PubMed
- Skinner PJ, Daniels MA, Schmidt CS, Jameson SC, Haase AT. Cutting edge: In situ tetramer staining of antigen-specific T cells in tissues. J Immunol. 2000;165:613–617. - PubMed
- Haanen JB, et al. In situ detection of virus- and tumor-specific T-cell immunity. Nature Med. 2000;6:1056–1060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI009484-32/AI/NIAID NIH HHS/United States
- T32 AG000080/AG/NIA NIH HHS/United States
- AG00080/AG/NIA NIH HHS/United States
- AI09484/AI/NIAID NIH HHS/United States
- R01 AI009484/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous